-
Lithium-ion batteries – electrolytes – solid & semi-solid
-
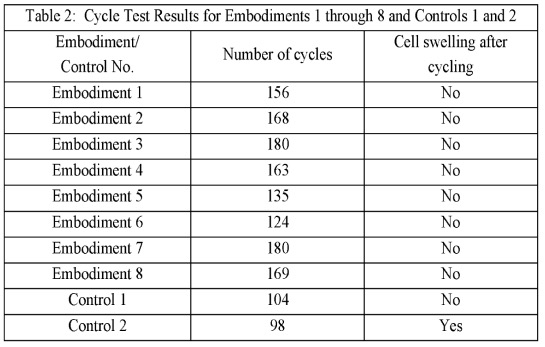
Pouch cells with lithium metal negative electrodes (thickness: ≈20 μm) and NMC811-based positive electrodes were built with varying cyclic sulfonamide-type salts as components
of liquid electrolytes as shown in the Figures and Tables below. Cells were cycled at 0.33 C charge / discharge until 80% capacity retention.
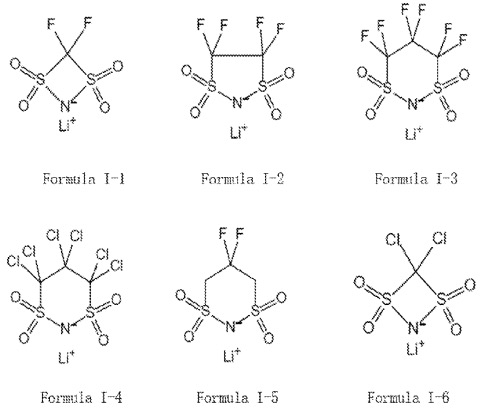
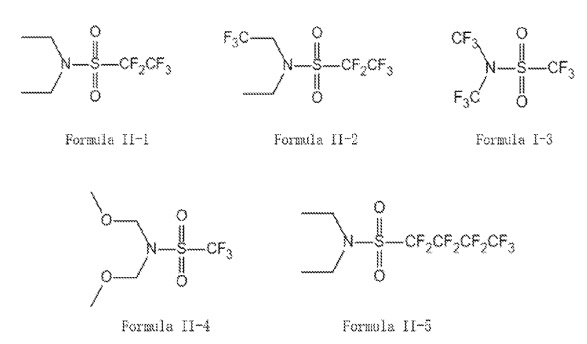
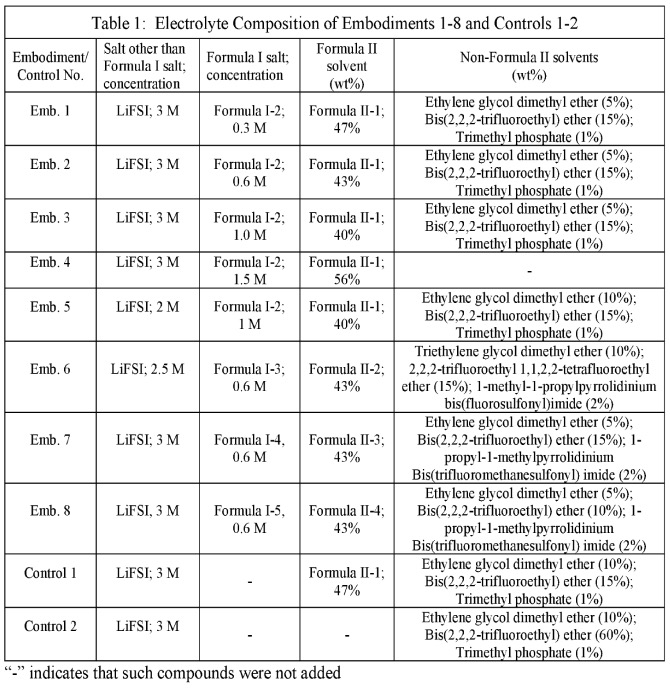
This patent application is covered in this category because molecules I-1 to I-6 and II-1 to II-5
may be useful for lithium metal electrode / electrolyte interface design in semi-solid Li-ion batteries.
As discussed in relation to earlier patent filings by SES, SES has developed a solid electrolyte layer that interfaces favorably with lithium metal electrodes
and might be deployed in semi-solid Li-ion battery cells.
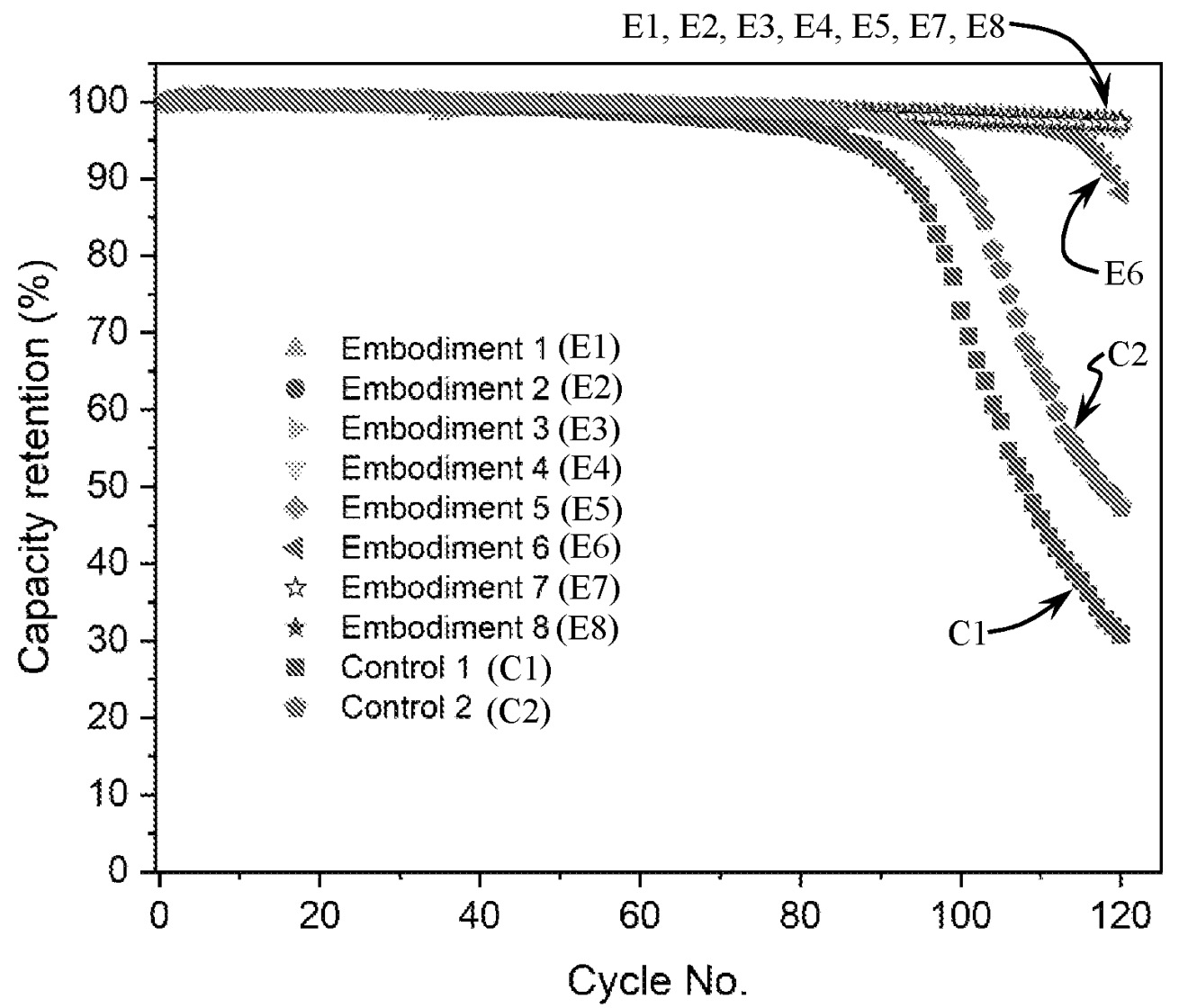
The cycling data below for liquid electrolyte cells illustrates once again that in the absence of a mechanically stable
solid electrolyte layer that faces the lithium metal electrodes,
lithium metal cells exhibit
an 'escalating capacity loss' problem upon reaching ≈90% capacity retention.
-
The premium version includes another two patent discussions, plus an Excel list with 50-100 commercially relevant recent patent families.
-
Lithium-ion batteries – negative electrode (excluding Li metal electrodes)
-
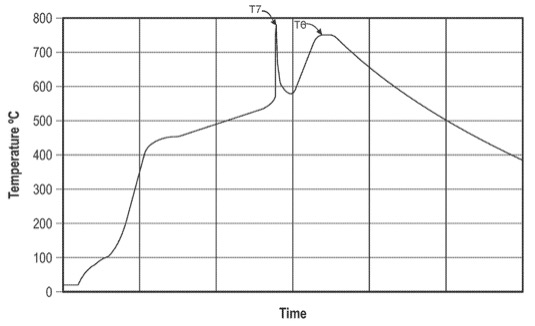
A de-alumination process was carried out on naturally occurring halloysite with the formula Si2Al2O5(OH)4 to obtain silica.
This silica material was combined with magnesium metal (≈100 μm particle size) and a moderator (NaCl or MgCl2, bimodal particle size distribution with peaks
at ≈40 μm and 180 μm), followed by a heat-treatment (see Figure) in a continuous rotary furnace that resulted in the triggering of magnesiothermic reduction
to reduce SiO2 to Si (peak, T7) and subsequent sintering at about 750°C to obtain porous Si. No etching step that involves HF (hydrofluoric acid) is necessary
(i.e. no need to etch off residual SiO2).
The BET specific surface area of the resulting Si material can be tuned between 20 m2/g (16 nm crystallite size) and 140 m2/g (5 nm crystallite size).
It is mentioned that this material can be CVD-coated with acetylene (CVD: chemical vapor deposition).
As shown on the website of Ionic Mineral Technologies,
the halloysite precursor that is sourced in a mine in Utah (USA) exhibits an
anisotropic nanotube morphology (average length: 500 nm, average diameter: 50 nm).
No morphology characterization data for the porous Si materials was identified in the patent.
-
The premium version includes another two patent discussions, plus an Excel list with 50-100 commercially relevant recent patent families.
-
Lithium-ion batteries – positive electrode
-
Manganese nitrate (0.235 equivalents), cobalt nitrate (0.045 equivalents), and nickel nitrate (0.63 equivalents) were dissolved in
water, followed by the addition of 4 M NaOH aqueous solution and 10.2 M NH3 ∙ H2O aqueous solution while stirring to produce a precipitate.
The precipitate was filtered, collected, and dried (110°C).
The precursor material prepared above was mixed with LiOH (1.1 equivalents) and Nb2O5
(0.045 equivalents), followed by a heat treatment (750°C, 9 h).
This process resulted in the Nb-doped, high-nickel, lithium-rich positive electrode active material
0.9 LiNi0.7Mn0.15Co0.05Nb0.1O2 ∙ 0.1 Li2MnO3.
In half-cells, the material exhibits a 0.5 C capacity of 273.0 mAh/g along with a capacity retention after 100 cycles of 90% (0.5 C charge / discharge,
2.5-4.8 V vs. Li+/Li).
This work illustrates a very promising energy density in an active material with comparably high Mn content (the cost of Mn is much lower than
Ni and Co).
Because of its comparably high voltage and Mn-content, the material might be of interest in particular in combination with semi-solid
and all-solid electrolytes (feasibility of electrochemical stability at high potential and reduced risk of Mn-leaching
/ deposition at the negative electrode).
-
The premium version includes another two patent discussions, plus an Excel list with 50-100 commercially relevant recent patent families.
-
Fuel cells (PEMFC / SOFC / PAFC / AEMFC) – electrochemically active materials
-
A platinum / carbon catalyst (40 mass% metal loading) and a ionomer were mixed with ethanol and water to prepare a cathode slurry (mass ratio: 1.2).
The slurry was introduced into a nanobubble generator (air supply at 100 bar). The pressurized slurry was discharged at atmospheric pressure,
creating a nanobubble-containing electrode slurry. This slurry was coated onto a substrate via casting to form an electrode layer.
The layer was freeze-dried (-130°C, 24 h), followed by secondary drying (80°C). The dried electrode layer was transferred
to the cathode side of a Nafion 211 membrane. Mercury intrusion porosimetry (MIP) confirmed the presence of micropores (size: ≈500 nm).
For the anode, a similar slurry was prepared, coated onto a substrate, and dried (80°C). The anode electrode layer was then transferred to
the polymer electrolyte membrane, forming a membrane-electrode assembly. A carbon gas diffusion layer (thickness: 300 μm) was bonded to both electrodes
to complete the unit cell.
Unit cell tests were made at a relative humidity of 50% and an operating temperature of 65°C, resulting in the cell potential / current density
curve shown below that illustrates improved performance as compared to a comparative example prepared without introducing nano-bubbles into the electrode slurries.
실시예: Example
비교예: Comparative Example
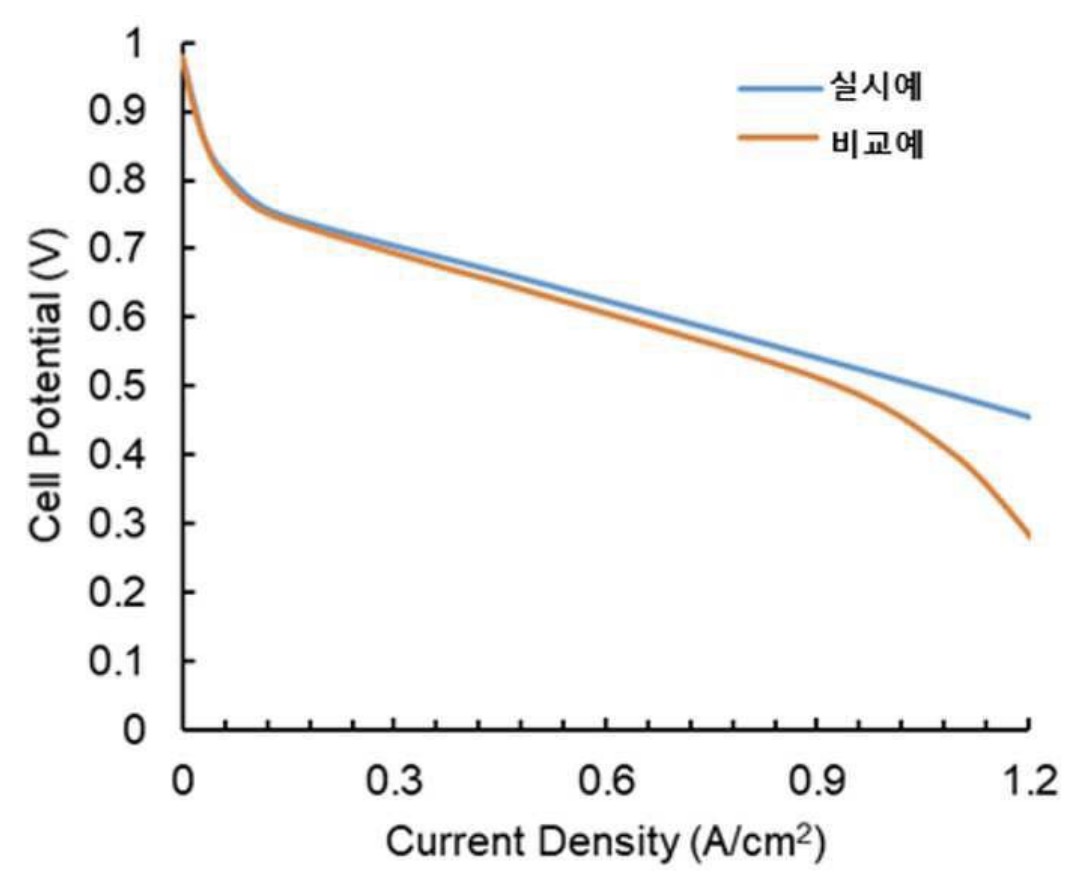
This work illustrates how the introduction of nano-bubbles into electrode slurries can substantially impact performance through
a change of the pore size distribution in electrochemically active layers.
-
The premium version includes another two patent discussions, plus an Excel list with 50-100 commercially relevant recent patent families.
-
Triweekly patent lists for other categories (Excel files are included for premium users)
-
- Lithium metal containing batteries (excluding Li-S, Li-Air): XLSX
-
- Lithium-ion batteries – electrolytes – liquid: XLSX
-
- Lithium-ion batteries – separators: XLSX
-
- Lithium-sulfur batteries: XLSX
-
- Metal-air batteries: XLSX
-
- Na-ion batteries: XLSX
-
Prior patent updates
-
2024-09-10
-
2024-08-20
-
2024-07-30
-
2024-07-09
-
2024-06-18
|