-
Lithium-ion batteries – electrolytes – solid & semi-solid
-
Solid electrolyte layers were prepared upon mixing of the tetrameric monomer below (≈10 mass%),
LiFSI (≈90 mass%, lithium bis(fluorosulfonyl)imide) and ≈0.5 mass% of one of the following 'additive salts':
LiBF4, LiPF6, Li2PO3F2,
Li2PO2F2, LiC4PO8F2,
LiC2PO4F4, Li[PFx(CyF2y+1−z)6-x] (1 ≤ x ≤ 5, 1 ≤ y ≤ 8, and 0 ≤ z ≤ 2y−1),
Li[BF2(C2O4)].
Cells with lithium metal negative electrodes, NMC811-based positive electrodes and the above solid electrolyte layer were assembled and
cured to trigger polymerization (60°C).
Comparative cells without 'additive salt' exhibit up to 20% reduced cycling stability.
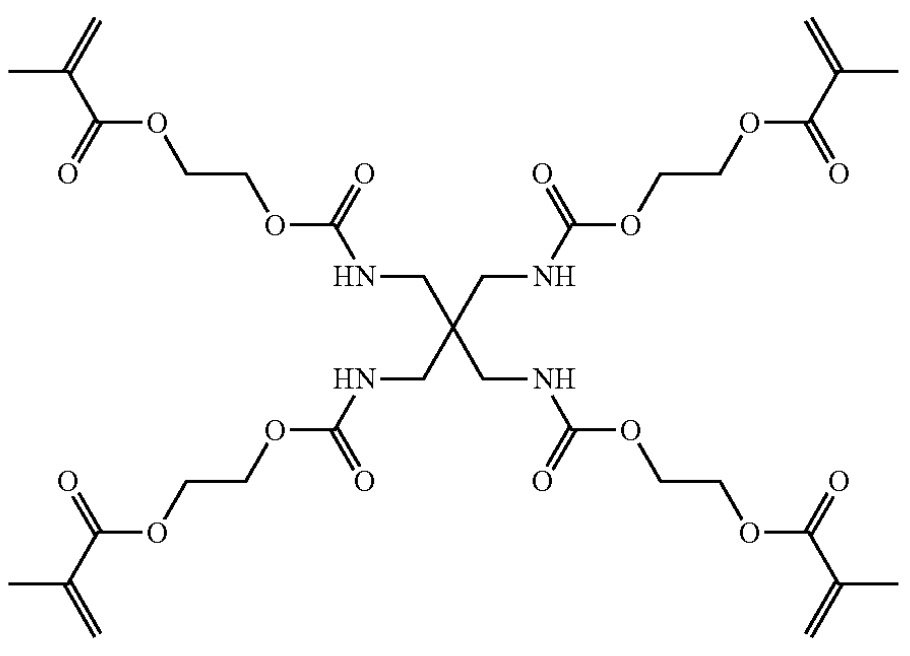
This patent application illustrates how Factorial evaluates cross-linked polymers with amide functional groups,
loaded with large quantities of Li-ion conducting salts.
A question is if more favorable mechanical characteristics have been obtained as compared to other
solid electrolyte layers that contain large concentrations of lithium salts, potentially because the polymer
contains amide functional groups that can undergo supramolecular binding interactions.
Another question is whether it has been confirmed if the
N-H groups
do not cause parasitic reactivity upon contact with lithium metal
(risk of hydrogen formation).
-
The premium version includes another two patent discussions, plus an Excel list with 50-100 commercially relevant recent patent families.
-
Lithium-ion batteries – positive electrode
-
A lithium-rich manganese-based active material was mixed with ammonium iron oxalate (2 mass%), followed by calcination (400°C, 3 h, nitrogen),
which results in a lithium-rich manganese-based positive electrode material with internal
iron ion-doping, three-dimensional channels near the surface with ionic conductivity, and oxygen vacancies.
In half-cells, the resulting material exhibits a 0.1 C discharge capacity of 267.7 mAh/g (charge to 4.55 V, discharge to 2.0 V vs. Li+/Li)
and a capacity retention after 100 cycles of 96.6% (0.1 C charge / discharge), as compared to 229.5 mAh/g and 75.2% for a comparative material that
was not treated with ammonium iron oxalate.
This work illustrates a that a post-treatment with ammonium iron oxalate can dramatically improve electrochemical characteristics.
In consideration that this post-treatment step appears to be highly cost-effective, it will be very interesting to see if it also improves
the performance other positive electrode active materials.
-
The premium version includes another two patent discussions, plus an Excel list with 50-100 commercially relevant recent patent families.
-
Lithium-ion batteries – negative electrode (excluding Li metal electrodes)
-
Submicron Si particles were obtained upon treating Si precursor powder in a 60 kW RF-ICP (radio frequency inductively coupled plasma)
process (formation of gaseous Si), followed by quenching and passivation of submicron Si particles with N2/O2 and mixing with petroleum-based pitch in a twin-screw extruder (230°C).
This powder was mixed with single-walled carbon nanotubes (SWCNT) and CMC in water, followed by drying (90°C, 2 h),
a heat treatment (960°C, 2 h, inert gas, volatilization of CMC, concentration of SWCNT near surface) and ball-milling (300 rpm, 1 h).
The resulting active material exhibits an Si / O / C matrix / SWCNT ratio of 45.3 : 5.4 : 49.1 : 0.1, an SiC / Si ratio of
0.058 and a BET specific surface area of 3.4 m2/g.
In half-cells, the material exhibits a capacity of 1,620 mAh/g. Upon mixing with graphite to obtain a capacity of 550 mAh/g,
a first cycle efficiency of 90.82% was observed along with an average coulombic efficiency in cycles 5-50 of 99.81% (0.5 C charge / discharge),
as compared to 1,622 mAh/g, 91.15% and 99.72% for a comparative active material mixture without CNT.
This work illustrates the pursuit by Umicore of further optimization of the carbon matrix that is combined with submicron Si particles
prepared through an RF-ICP treatment.
It appears that formation of the right quantity of SiC (silicon carbide) was optimized by adjusting heat treatment conditions, and
that it was found that the presence of a minor amount of SiC results in a positive effect on electrochemical performance.
-
The premium version includes another two patent discussions, plus an Excel list with 50-100 commercially relevant recent patent families.
-
Fuel cells (PEMFC / SOFC / PAFC / AEMFC) – electrochemically active materials
-
An aqueous solution was prepared with Nafion (Fujifilm Wako Pure Chemical Industries, DE2020) and calcium chloride (≈20% calcium ions relative to
the quantity of protons on Nafion) by using an ultrasonic homogenizer (2 h, room temperature, Nippon Seiki Seisakusho, US-150E). Partial replacement of protons with calcium ions leads to
a change of the radius of gyration of the polymer through supramolecular coordination interactions.
This solution was mixed with a catalyst (Pt on porous carbon) slurry in ethanol / water using a dispersing machine (Seiwa Giken, RS-05W) and
an ultrasonic homogenizer (same as above), followed by screen printing on a PTFE sheet and a heat treatment (110°C, 1 h).
In a power generation test, an increased voltage (10-15 mV) was observed upon use as anode or cathode catalyst at 0.3 A/cm2 / 60°C.
This work suggests that the relatively straightforward addition of Ca2+-ions to ionomers allows for a slightly improved power performance in PEMFC.
-
The premium version includes another two patent discussions, plus an Excel list with 50-100 commercially relevant recent patent families.
-
Triweekly patent lists for other categories (Excel files are included for premium users)
-
- Lithium metal containing batteries (excluding Li-S, Li-Air): XLSX
-
- Lithium-ion batteries – electrolytes – liquid: XLSX
-
- Lithium-ion batteries – separators: XLSX
-
- Lithium-sulfur batteries: XLSX
-
- Metal-air batteries: XLSX
-
- Na-ion batteries: XLSX
-
Prior patent updates
-
2024-08-20
-
2024-07-30
-
2024-07-09
-
2024-06-18
-
2024-05-28
|