-
Lithium-ion batteries – electrolytes – solid & semi-solid
-
This patent application illustrates how PTFE (polytetrafluoroethylene)-based fibrillization can be employed to simultaneously produce and laminate
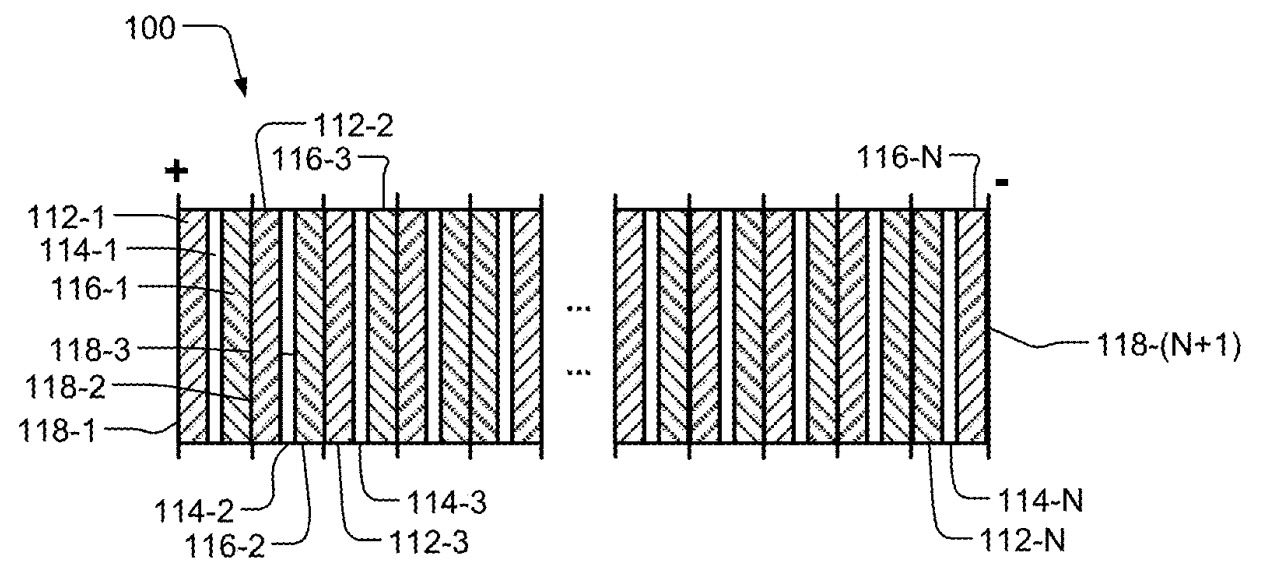
electrode and (sulfide or halide) electrolyte layers to obtain bipolar cells (see Figure).
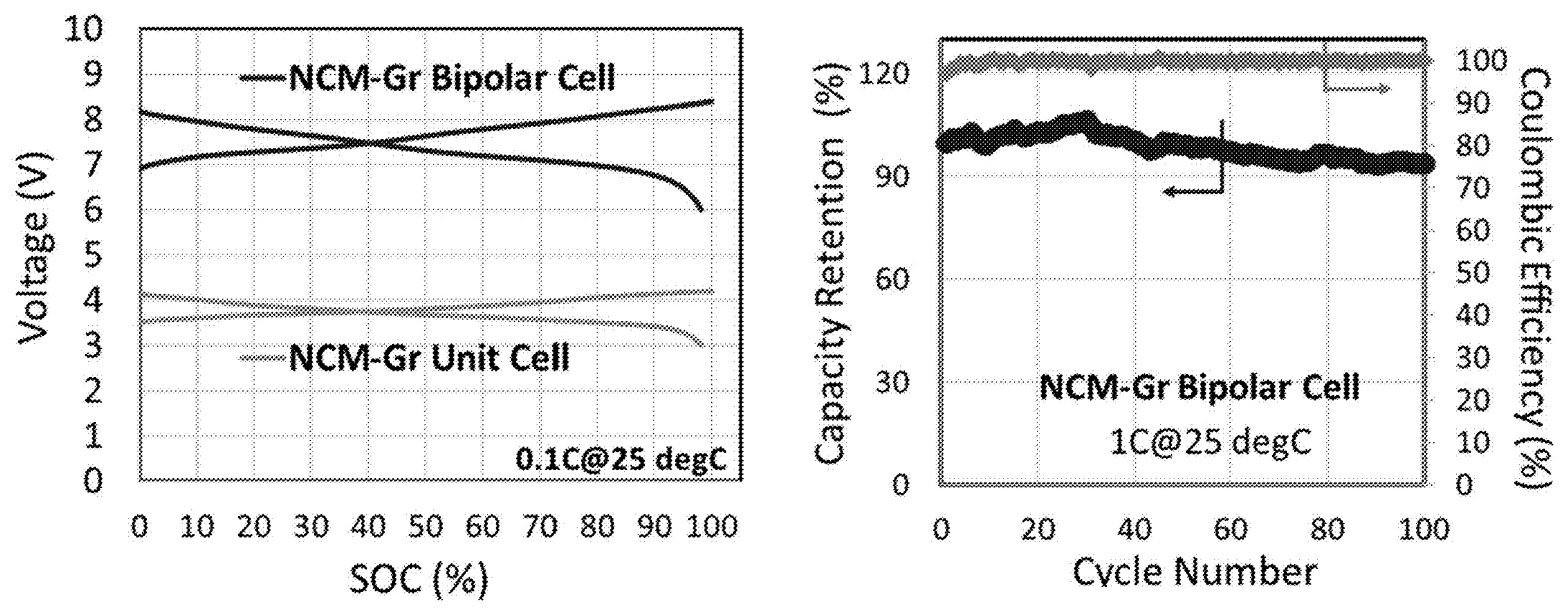
Although no detailed examples are included in this patent application, electrochemical cycling
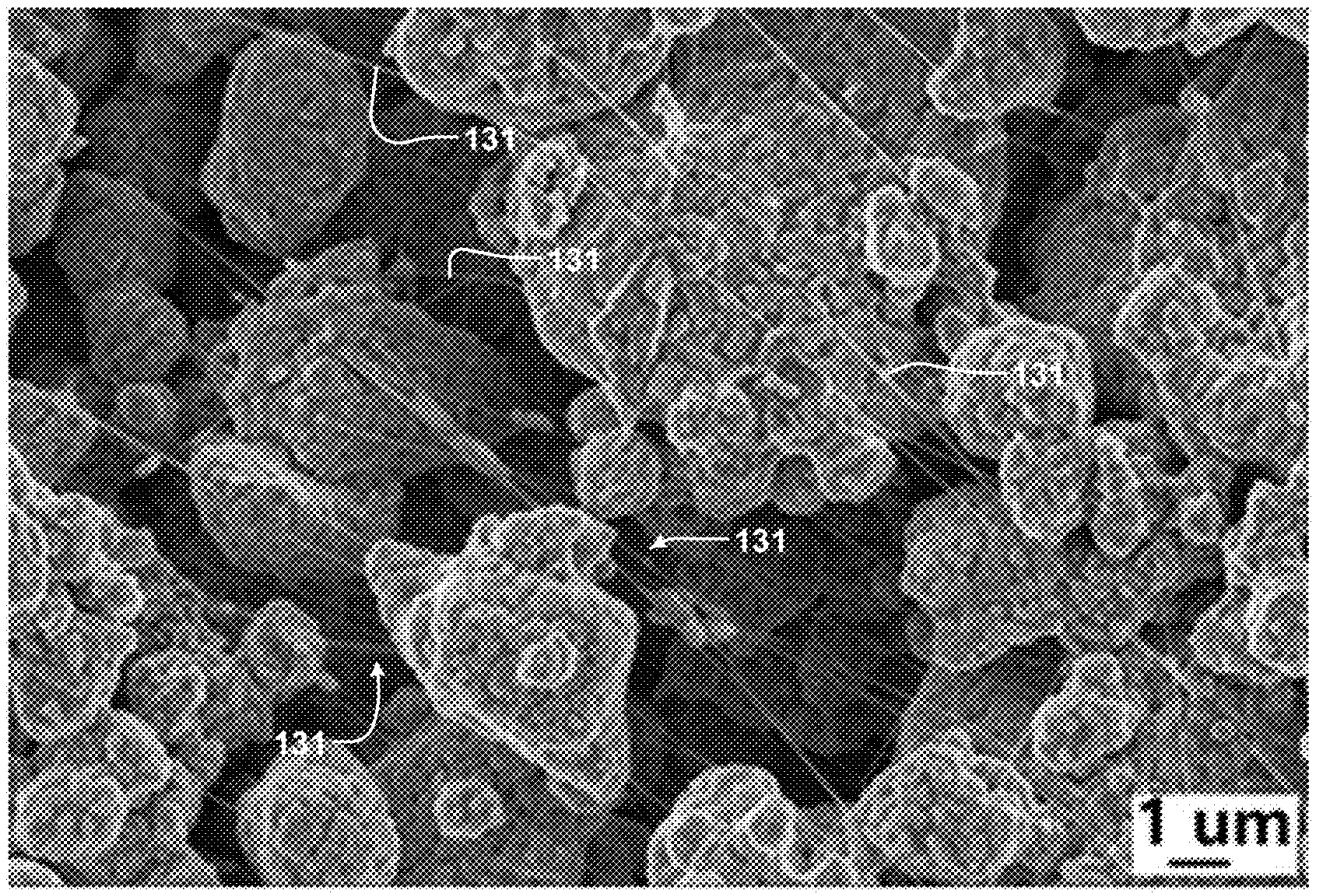
data for a NMC / graphite-based bipolar cell (2 stacked electrode pairs) and the SEM image of an NMC-based positive electrode are shown
in the Figure below.
100: bipolar battery cell
112: cathode electrodes
114: solid electrolyte
116: anode electrodes
118: bipolar current collectors (composition not disclosed)
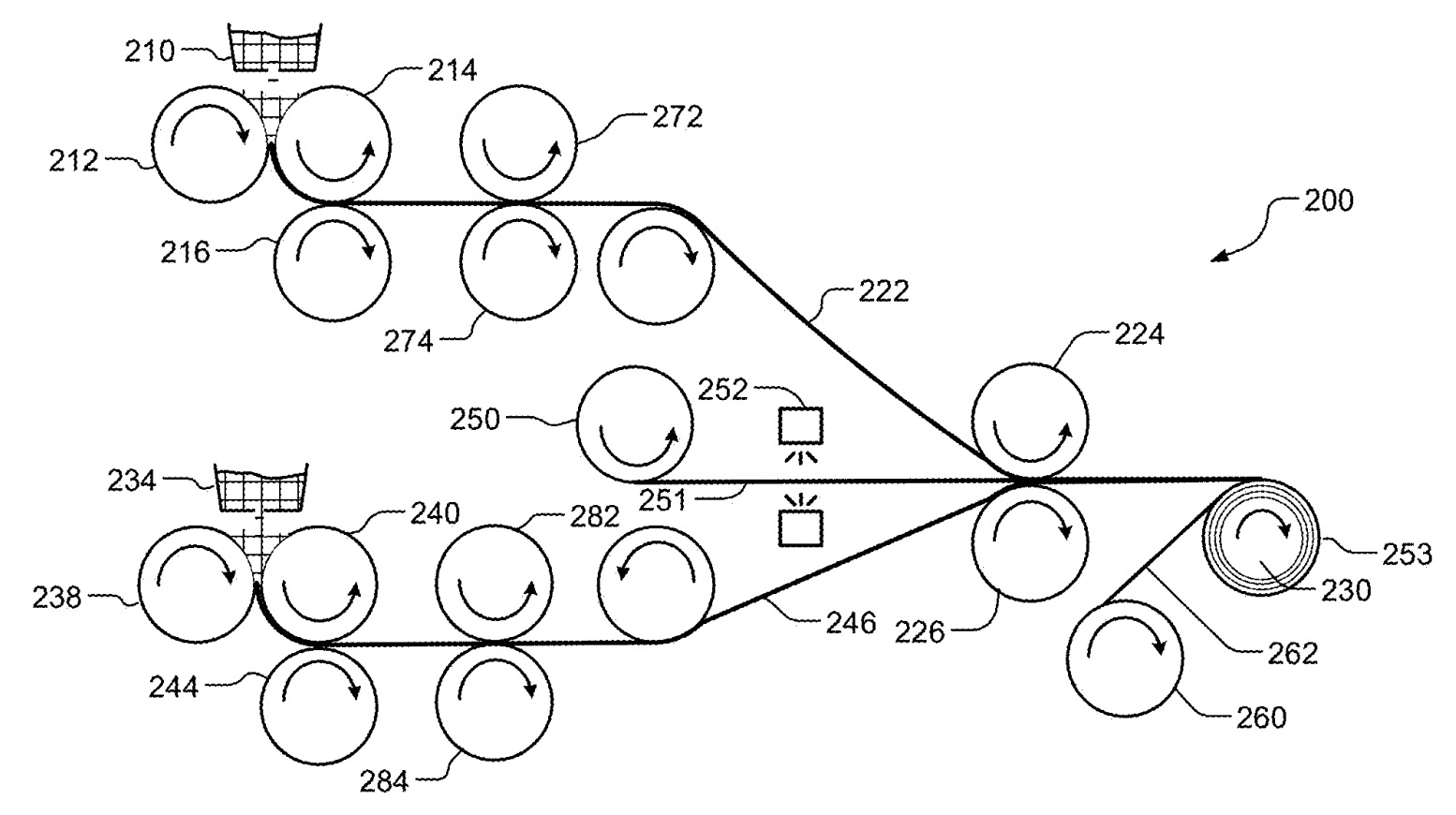
210: first source
212, 214, 216: rollers
222: cathode electrode
224, 226: rollers
230: roll
234: second source
238, 240, 244: rollers
246: anode electrode
250: roll
251: current collector
252: sprayer
253: laminate
260: roll
262: separating layer
272, 274, 282, 284: additional rollers
This work illustrates how dry fibrillization allows for the efficient formation of bipolar cell architectures.
It will be very interesting to follow further upscaling efforts for both the EV and the stationary energy storage sector, which both exhibit
the urgent need for reduced costs at large production scale.
-
The premium version includes another two patent discussions, plus an Excel list with 50-100 commercially relevant recent patent families.
-
Lithium-ion batteries – positive electrode
-
A hydroxide precursor with the transition metal composition Ni0.96Mn0.01Co0.03 was mixed
with LiOH (lithium to transition metal ratio of 0.99), Al2O3 (700 ppm) and
Y0.3Zr0.7O1.85 (0.05 mol% Y, 0.117 mol% Zr),
followed by a
heat-treatment (805°C, 10 h, then 720°C, 5 h, oxygen flow) and bead milling in an aqueous cobalt solution (concentration: 0.5 mol% Co with
respect to total transition metal content, 13 h).
This material was mixed with LiOH (lithium to transition metal ratio of 0.99, some Li was probably lost during wet milling),
nano-cobalt oxide (Co3O4) powder (1.5 mol% Co), Nb2O5 (500 ppm) and Al2O3
powder (500 ppm), followed by a heat treatment (710°C, 12.5 h, oxygen atmosphere).
The resulting NMC active material exhibits a Ni / Co / Zr / Y molar ratio of 93.6 : 4.9 : 0.095 : 0.037 according to
an ICP (inductively coupled plasma) measurement (not listed: Mn content),
and a Coedge / Cocenter ratio of 1.84 according to CS-EDS (cross-sectional energy dispersive spectrocopy).
In full cells (graphite-based negative electrodes), this material exhibits 1,363 cycles (up to 80% capacity cutoff, 1 C charge / discharge).
This work illustrates a nickel-rich NMC material with favorable cycling stability, based on a multi-doping approach (Zr, Y, Al, Nb)
and the employment
of a Co-gradient in particles.
-
The premium version includes another two patent discussions, plus an Excel list with 50-100 commercially relevant recent patent families.
-
Lithium-ion batteries – negative electrode (excluding Li metal electrodes)
-
Negative electrodes were fabricated by coating a slurry of carbon-coated Si microparticles (Si diameter: ≈4 μm, carbon coating
thickness: <10 nm), polyimide, and a carbon additive
(such as carbon nanotubes) on a PP (polypropylene) film, followed by drying, followed
by a heat treatment (600-1,250°C, 1-3 h). The PP film was volatilized in this process with ≈2% char residue.
Claims include that the Si source is quartz (i.e. metallurgical Si is probably used).
The carbonized electrode was subsequently laminated on a copper current collector foil coated with
a polyamide-imide adhesive layer.
Full cell cycling tests (NMC811-based positive electrodes) illustrate an improved cycling stability for cells with carbon-coated Si microparticles
(404),
as compared to cells with uncoated Si microparticles (402).
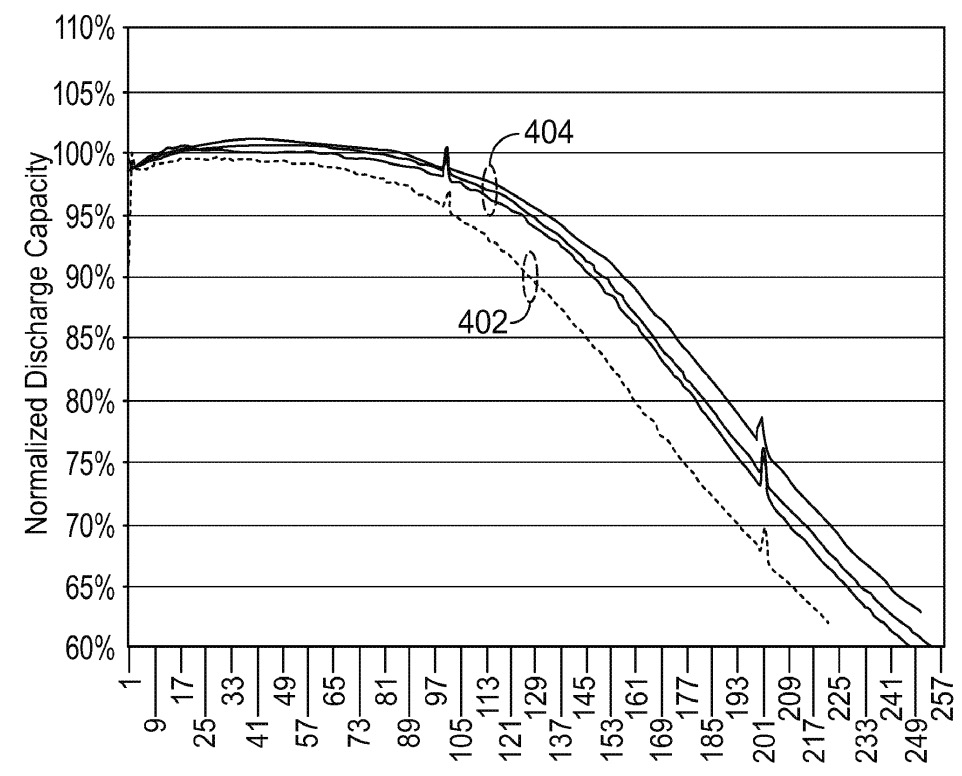
This work illustrates how Enevate might employ carbon-coated Si microparticles in its electrodes, probably obtained through
CVD (chemical vapor deposition, such as with methane, ethene or acetylene) on metallurgical Si microparticles.
It is very interesting that PP films might be employed as sacrificial support layer during electrode carbonization, avoiding
the need for up-scaling the electrode peel-off process used typically at lab scale.
-
The premium version includes another two patent discussions, plus an Excel list with 50-100 commercially relevant recent patent families.
-
Fuel cells (PEMFC / SOFC / PAFC / AEMFC) – electrochemically active materials
-
Pt-supported carbon was added to acetonitrile, followed by the addition of an ionic liquid (1-alkyl-3-methylimidazolium bis(trifluoromethanesulfonyl)imide)
to infuse the mesopore volume of the
Pt-supported carbon.
After ultrasonic dispersion (30 min) and stirring (overnight), acetonitrile was removed with an evaporator,
resulting in ionic liquid-impregnated, Pt-supported carbon.
This material was added to water, followed by ultrasonic stirring and the addition of sodium hydroxide.
A mixed solution of TEOS (tetraethoxysilane) and ethanol was then added, followed by stirring (2 h).
The material was separated using a centrifuge and dried (80°C, 6 h), yielding ionic liquid-infused,
silica-coated, Pt-supported carbon with an XRD (X-ray diffraction) determined Pt particle crystallite size (1,1,1) of 6.4 nm (see Figure).
In a rotating disk electrode measurement, the material exhibits an an initial activity of 17.5 A/g-Pt and of 14.6 A/g-Pt
after aging (1,200 cyclic voltammetry
cycles of 0.6 to 1.21 V vs. RHE, reversible hydrogen electrode).
(a): explanatory diagram
(b), (c): schematic cross-sections
1: silica-coated catalyst particles impregnated with ionic liquid
2: conductive support (carbon)
3: metal particles (platinum)
4: silica membrane
5: ionic liquid
20: catalyst particle
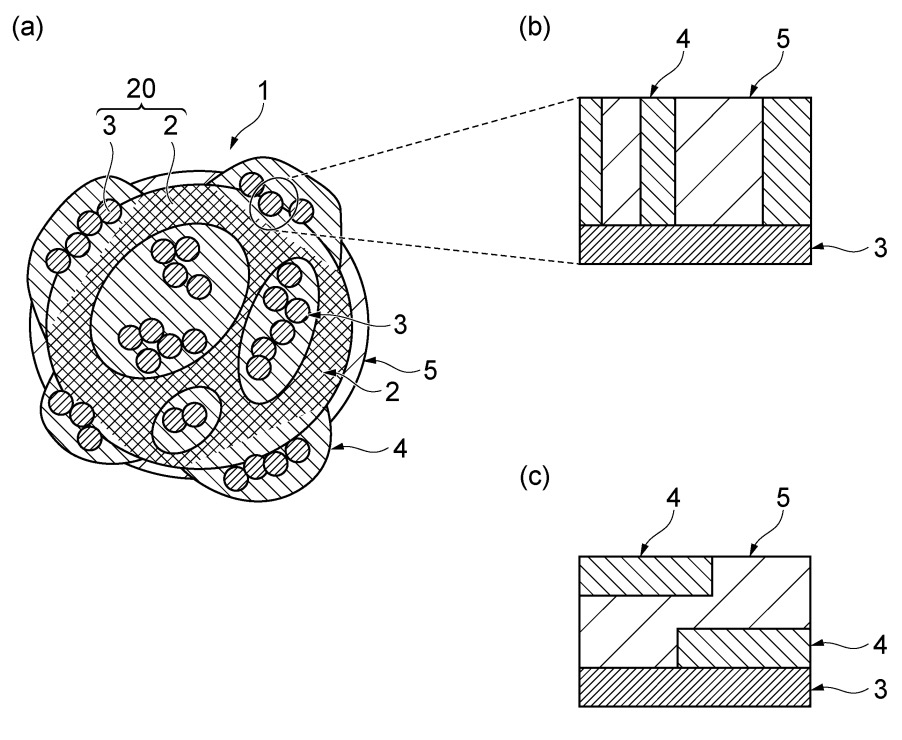
This work illustrates how ionic liquids can enable a favorable Pt distribution and pore size distribution
of a Pt/carbon catalyst / silica composite particle.
-
The premium version includes another two patent discussions, plus an Excel list with 50-100 commercially relevant recent patent families.
-
Triweekly patent lists for other categories (Excel files are included for premium users)
-
- Lithium metal containing batteries (excluding Li-S, Li-Air): XLSX
-
- Lithium-ion batteries – electrolytes – liquid: XLSX
-
- Lithium-ion batteries – separators: XLSX
-
- Lithium-sulfur batteries: XLSX
-
- Metal-air batteries: XLSX
-
- Na-ion batteries: XLSX
-
Prior patent updates
-
2024-05-07
-
2024-04-16
-
2024-03-26
-
2024-03-05
-
2024-02-13
|