-
Lithium-ion batteries – electrolytes – solid & semi-solid
-
A layer of Cu6Sn5OX or SnOX (X: varying O content that reflects surface oxidation)
was deposited on an LLZTO (Li6.5La3Zr1.5Ta0.5O12) pellet by rubbing it over excess Cu and Sn powder,
blowing off metal powder, followed by rapid melting between graphite plates (1,100°C) and rapid quenching (≈103 °C/min).
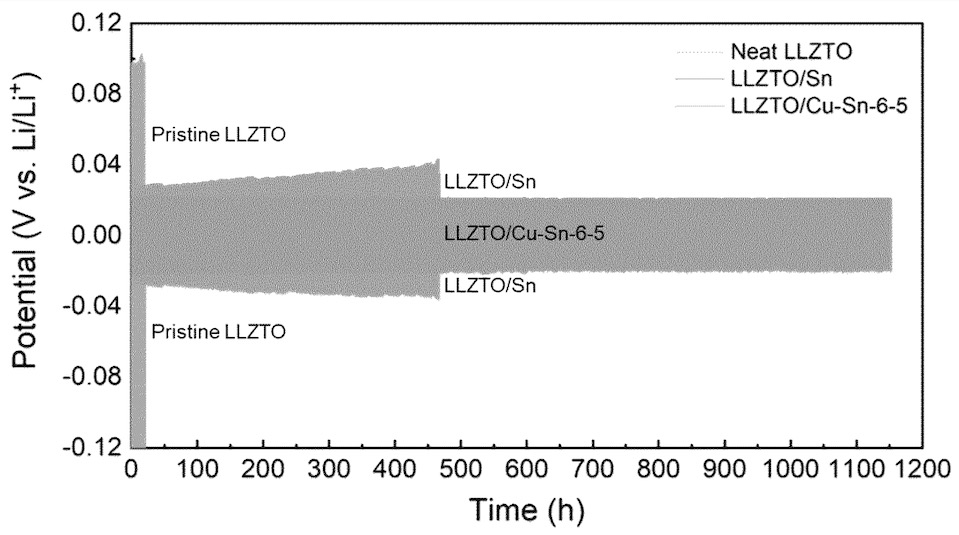
As shown in the top Figure, a significantly reduced polarization was observed upon cycling in symmetric cells at 0.2 mA/cm2
in the presence of metal interface layers.
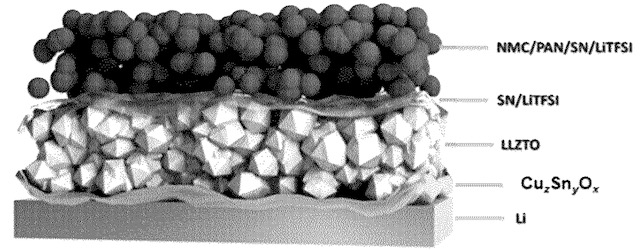
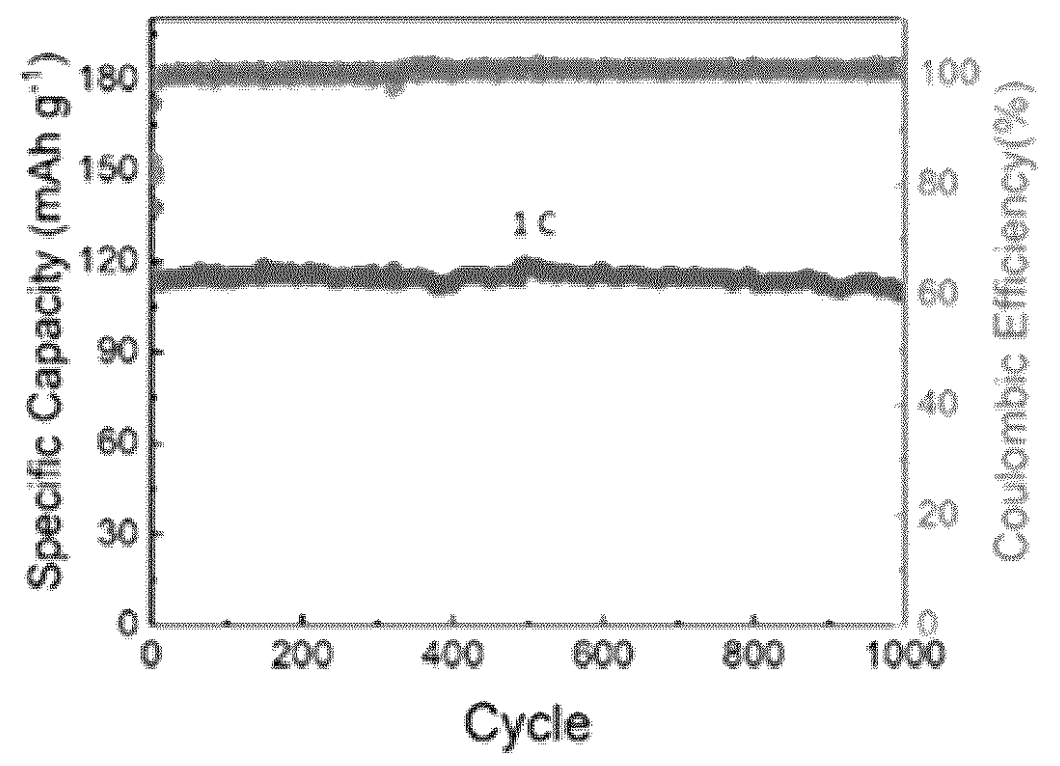
Cells as shown in the middle Figure were built and cycled at various C rates (bottom Figure).
PAN: polyacrylonitrile
SN: succinonitrile
LiTFSI: lithium bis(trifluoromethane)sulfonimide
This work indicates that an electrically and ionically conducting metal alloy layer between lithium metal negative electrodes
and oxide electrolyte layers leads to a substantially reduced
internal resistance and improved cycling stability.
This approach could allow for higher defect-tolerance in oxide electrolyte films of lithium metal cells,
which in turn could allow for reduced oxide electrolyte film production costs (faster sintering).
-
The premium version includes another two patent discussions, plus an Excel list with 50-100 commercially relevant recent patent families.
-
Lithium-ion batteries – positive electrode
-
Ni0.8Co0.1Mn0.1(OH)2 was mixed with lithium hydroxide (1.07 : 1 molar ratio),
followed by sintering (700°C, 10 h).
This material was blended with water and a mixture of
P123
templating / micelle formation agent and lithium metaaluminate (LiAlO2) was added.
The solution's pH was adjusted to 7 using a 50 volume% nitric acid solution, followed by stirring (50°C, 1 h),
standing (48 h), and suction filtration. After washing with deionized water and absolute ethanol,
the product was dried (100°C, vacuum, 12 h) and calcined in a muffle furnace (600°C, 4 h) to obtain
porous alumina-coated NMC811.
In half-cell tests, the material exhibits a discharge capacity of 236.5 mAh/g along with a capacity retention after 100 cycles (1 C charge / discharge) of 96.7%,
as compared to 220.6 mAh/g and 94.8% for a comparative material (incorporation of alumina coating through dry mixing of alumina powder with NMC811).
It is argued that the coating allows for avoidance of undesired residual Li surface compounds.
This work suggests that use of the sacrificial mesopore former P123 and LiAlO2 on a Li-deficient NMC811 intermediate
leads to a very favorable porous alumina coating while fully lithiating NMC811,
allowing for substantially improved capacity and capacity retention.
It therefore appears that the introduction of lithium into lithium-deficient NMC through the use of LiAlO2 as Al2O3
coating precursor is highly beneficial, together with
the introduction of alumina porosity.
-
The premium version includes another two patent discussions, plus an Excel list with 50-100 commercially relevant recent patent families.
-
Lithium-ion batteries – negative electrode (excluding Li metal electrodes)
-
A silicon-based anode material with a hollow structure was produced.
A mixture of styrene, methyl methacrylate, butyl acrylate, and methacrylic acid monomers was polymerized (85°C, nitrogen atmosphere).
A silicate shell was then formed around the polymer core by adding sodium silicate.
Magnesium metal and sodium chloride were mixed with these particles, followed by a heat-treatment (660°C, argon atmosphere),
creating a hollow structure.
The product was dispersed in an ethanol / water mixture,
treated with hydrochloric acid to remove MgO and Mg2Si, resulting in the hollow silicon-based anode material as shown in the SEM
image below.
In half-cells, a capacity retention of 86% was observed after 50 cycles along with an electrode expansion of 14% (particles with
285 nm average diameter).
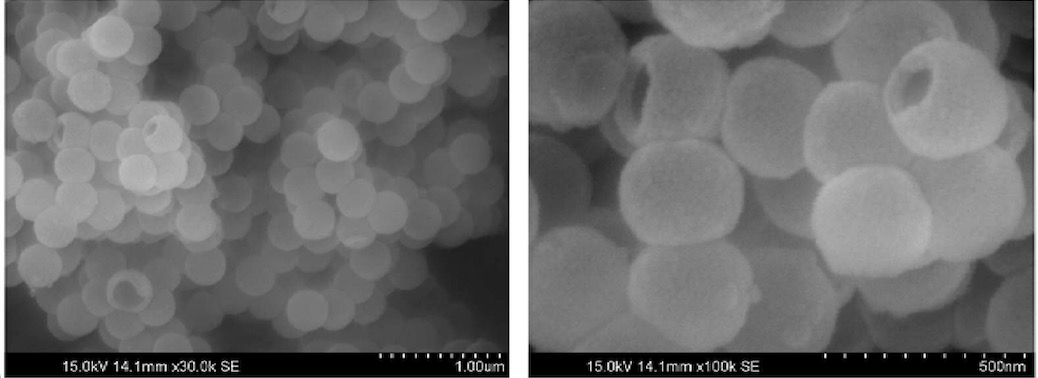
This work illustrates the uniform formation of hollow Si nano-spheres.
It will be interesting to see performance in full cells and irreversible capacity losses with various electrolytes (not disclosed).
-
The premium version includes another two patent discussions, plus an Excel list with 50-100 commercially relevant recent patent families.
-
Fuel cells (PEMFC / SOFC / PAFC / AEMFC) – electrochemically active materials
-
A mesoporous Sb-SnO2 catalyst support material was prepared.
Initially, connected starburst silica (CSS) was prepared by mixing cetyltrimethylammonium chloride,
methanol, ethylene glycol, NaOH, and tetraethoxysilane (TEOS) at 50°C, followed by drying (45°C) and a heat treatment
to form radial pores (multi-step temperature program up to 900°C, under nitrogen).
This CSS was then infiltrated with furfuryl alcohol, polymerized, and carbonized (500°C, under nitrogen),
followed by etching with 12% HF solution to remove silica, followed by drying (45°C) to obtain a connected starbust carbon (CSC) material
with a BET specific surface area of 2,122 m2/g.
Sb-SnO2/carbon was synthesized by dissolving SbCl3 and SnCl2 in hydrochloric acid,
then adding CSC and diluting with water, filtration, drying (85°C) and a heat-treatment (320°C, 24 h, air)
to create blue beaded mesoporous Sb-SnO2
(2.5 atom% Sb, 5.8 nm pore diameter 0.218 cm3/g pore volume).
Pt nanoparticles were deposited on this substrate via a colloid method involving NaOH/ethylene glycol and H2PtCl6/ethylene
glycol solutions
to achieve a Pt/Sb-SnO2 material with 20 mass% Pt loading. To make the catalyst water-repellant, it was mixed with Cytop fluoropolymer
(CTL-109AE, AGC Corp.)
and CT-Solv dilution solvent
(100E, AGC Corp.),
followed by vacuum drying (150°C, 1 h).
In the context of a PEMFC cathode layer, a current density of about 270 A/g was observed at 0.84 V at 80% relative humidity.
This work illustrates how a mesoporous Sb-SnO2 catalyst support on which 20 mass% Pt is deposited exhibits
well-balanced characteristics in terms of achieving favorable mass and electron transfer, for which water-repellant
features of the catalyst are crucial (to avoid channel blocking by water droplets).
-
The premium version includes another two patent discussions, plus an Excel list with 50-100 commercially relevant recent patent families.
-
Triweekly patent lists for other categories (Excel files are included for premium users)
-
- Lithium metal containing batteries (excluding Li-S, Li-Air): XLSX
-
- Lithium-ion batteries – electrolytes – liquid: XLSX
-
- Lithium-ion batteries – separators: XLSX
-
- Lithium-sulfur batteries: XLSX
-
- Metal-air batteries: XLSX
-
- Na-ion batteries: XLSX
-
Prior patent updates
-
2024-01-23
-
2024-01-02
-
2023-12-12
-
2023-11-21
-
2023-10-31
|