-
Lithium-ion batteries – electrolytes – solid & semi-solid
-
A continuous process is described in which a solid electrolyte melt is converted into a film
with the help of height limiting element 213 (see Figure) at 1,100°C (P1). The film is gradually cooled down
to 600°C (P2) on molten tin (211). Finally, the solidified electrolyte film is transported on a conveyor belt.
No information on electrolyte composition and electrochemical performance was disclosed.
201: electrolyte melt bath
203: metal molten pool
205: tempering element
207: electrolyte melt
209: rotary roller
211: molten metal (higher density than solid electrolyte, probably tin)
213: height limiting element
215: solid electrolyte
217: fan
219: conveyor belt
P1: first point (temperature: 1,100°C)
P2: second point (temperature: 600°C)
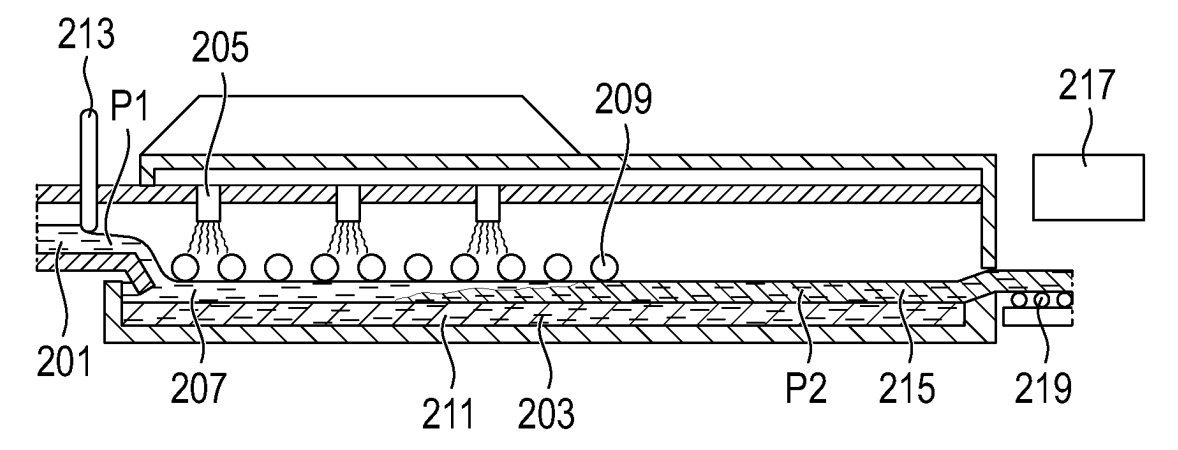
Although no electrolyte type was disclosed, it is probable that this process was designed for the formation of
oxide electrolyte films. In this sense, this patent filing might relate to Volkswagen's solid-state battery development partnership with QuantumScape.
This approach is very interesting in the sense that it appears principally plausible that a film with few defects and internal
strain can probably be formed.
Commercial viability of this process depends on the exact rate at which thin films can be cooled down
(which defines the throughput rate / oven length), and on the achievable film thickness (ideally ≤10 μm) and uniformity.
-
The premium version includes another two patent discussions, plus an Excel list with 50-100 commercially relevant recent patent families.
-
Lithium-ion batteries – positive electrode
-
LITHIUM-RICH NICKEL-RICH POSITIVE ELECTRODE ACTIVE MATERIAL
Applicant:
UMICORE / CENTRE NATIONAL DE LA RECHERCHE SCIENTIFIQUE / COLLEGE DE FRANCE / SORBONNE UNIVERSITE /
WO 2023247528 A1
Mixed metal solutions were prepared by dissolving lithium acetate dihydrate and nickel acetate tetrahydrate in ethanol,
and in parallel ammonium heptamolybdate in water. The two solutions were then combined and the resulting solution was heated at 80°C
to form a gel-type mixture. The mixture was then dried (120°C, oxygen flow, 8 h) and ground.
As shown in the Table below, a favorable discharge capacity and relative capacity (capacity retention after 101 cycles) was observed in half-cells,
for example in EX2 (CEX: comparative examples).
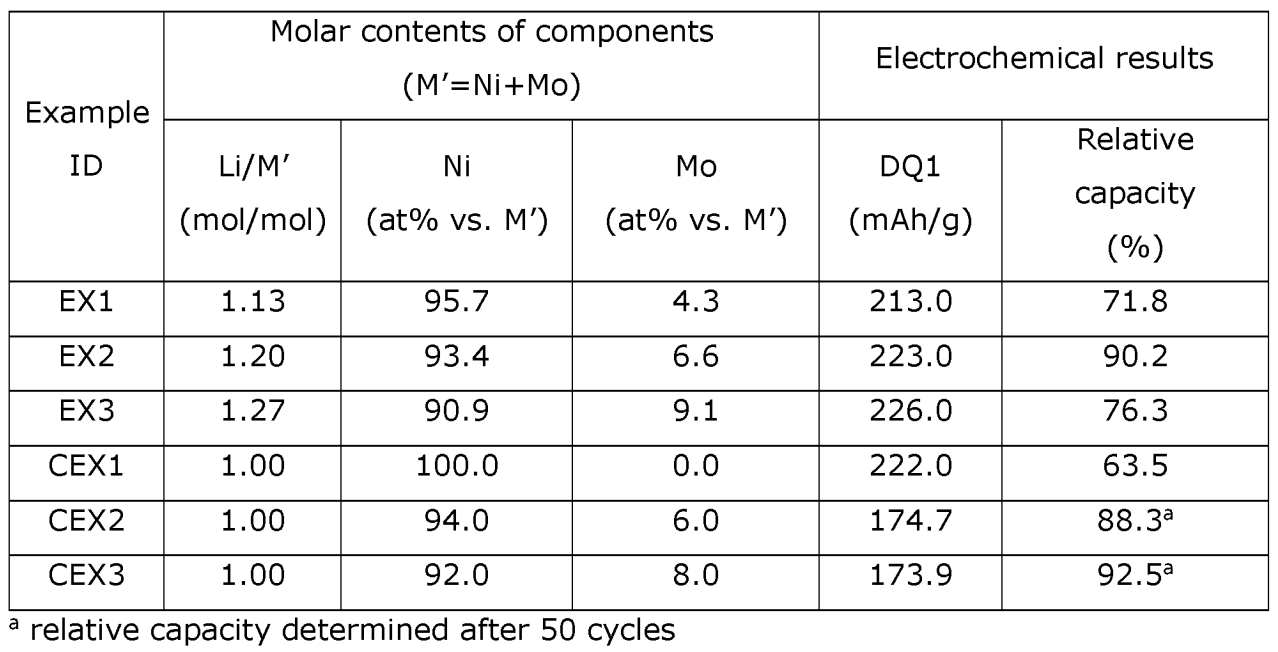
This work illustrates how overlithiated Ni-Mo oxides exhibit promising electrochemical characteristics upon use of a comparably
straightforward synthesis protocol.
Further doping, coating and up-scaling work is necessary to optimize performance in full cells.
-
The premium version includes another two patent discussions, plus an Excel list with 50-100 commercially relevant recent patent families.
-
Lithium-ion batteries – negative electrode (excluding Li metal electrodes)
-
A porous silicon-carbon composite active material was prepared in 3 steps:
-
Nano-silicon, liquid acrylate resin precursor, and a photoinitiator 1173 (probably 2-hydroxy-2-methyl-1-phenylpropanone) were mixed in a
mass ratio of 100 : 10 : 0.1 using a high-speed mixer.
This mixture was atomized into droplets and photo-cured using a high-pressure mercury lamp
(300-600 nm wavelength, 30 mW/cm2 intensity, 0.3 s exposure, 0.9 mJ/cm2 incident light).
The resulting first precursor has a pore structure with micropores (<2 nm) and mesopores (2-5 nm),
with micropores constituting 85% of the structure.
-
Washing with ethanol to remove uncured resin precursor.
-
Sintering under nitrogen (ramp at 1°C/min to 1,000°C, hold for 3 h).
The resulting material (see Figure with SEM image) exhibits a D50 diameter of 2 μm, a porous Si-carbon core (Si particle diameter: 20 nm), and a carbon coating.
In half-cell tests, the material exhibits a discharge capacity of 2,033 mAh/g, a first cycle efficiency of 90.2%, and a capacity retention after
50 cycles of 92.2% (0.2 C charge / discharge).
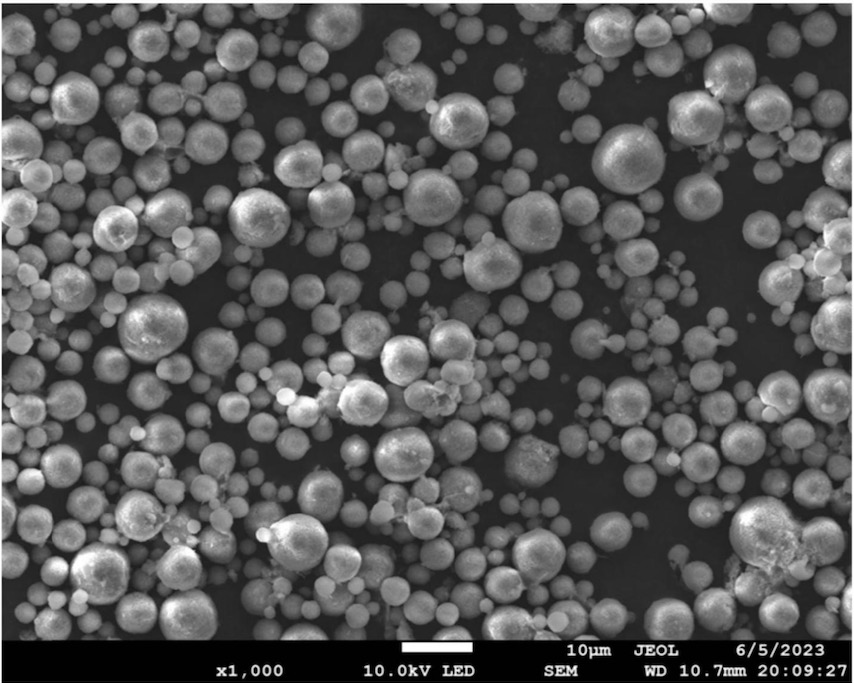
This work illustrates how a porous carbon scaffold can be generated upon partially reacting acrylate precursor using photo-curing in the
presence of Si nanoparticles, followed by washing away unreacted precursor.
Despite the porosity, a carbon surface layer is formed upon sintering that likely leads to a comparably
low BET specific surface area of the resulting microparticles, as indicated by a first cycle efficiency of >90% in half-cells.
This work illustrates how tailored carbon porosity can be generated by controlling the extent of photo-irradiation during polymerization. The low-cost
generation of carbon scaffolds with tailorable porosity is important in a variety of silicon-based active material development efforts.
-
The premium version includes another two patent discussions, plus an Excel list with 50-100 commercially relevant recent patent families.
-
Fuel cells (PEMFC / SOFC / PAFC / AEMFC) – electrochemically active materials
-
A functional layer with comparably low Pt content in combination with carbon and ionomer was introduced between the cathode layer and
the membrane (see Figure, left: scheme, middle: SEM image, right: EDX mapping image of Pt)
in a PEMFC cell.
Although no specific electrochemical data was presented, it was argued that favorable performance and longevity can be achieved with this cell
design based on reduced overall Pt content.
SEM: scanning electron microscopy
EDX: energy-dispersive X-ray spectroscopy
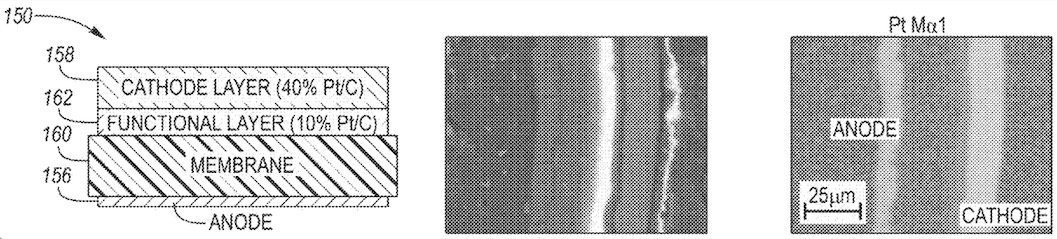
This work illustrates a complementary approach towards reducing platinum content in PEMFC, as compared to the reduction of platinum
content in the catalyst particles.
-
The premium version includes another two patent discussions, plus an Excel list with 50-100 commercially relevant recent patent families.
-
Triweekly patent lists for other categories (Excel files are included for premium users)
-
- Lithium metal containing batteries (excluding Li-S, Li-Air): XLSX
-
- Lithium-ion batteries – electrolytes – liquid: XLSX
-
- Lithium-ion batteries – separators: XLSX
-
- Lithium-sulfur batteries: XLSX
-
- Metal-air batteries: XLSX
-
- Na-ion batteries: XLSX
-
Prior patent updates
-
2024-01-02
-
2023-12-12
-
2023-11-21
-
2023-10-31
-
2023-10-10
|