-
Lithium-ion batteries – electrolytes – solid & semi-solid
-
An ion conductivity of 4.5 × 10-5 S/cm at 25° C was achieved for defect-doped LiCl ∙ FeOCl powder (see Figure below,
circles: defect-doped, triangles: undoped),
produced by heating Fe2O3 and FeCl3 to >200°C, followed by
defect doping (either heating or pressing in contact with a polar solvent, or heating in contact with a
reducing atmosphere, such as H2/Ar).
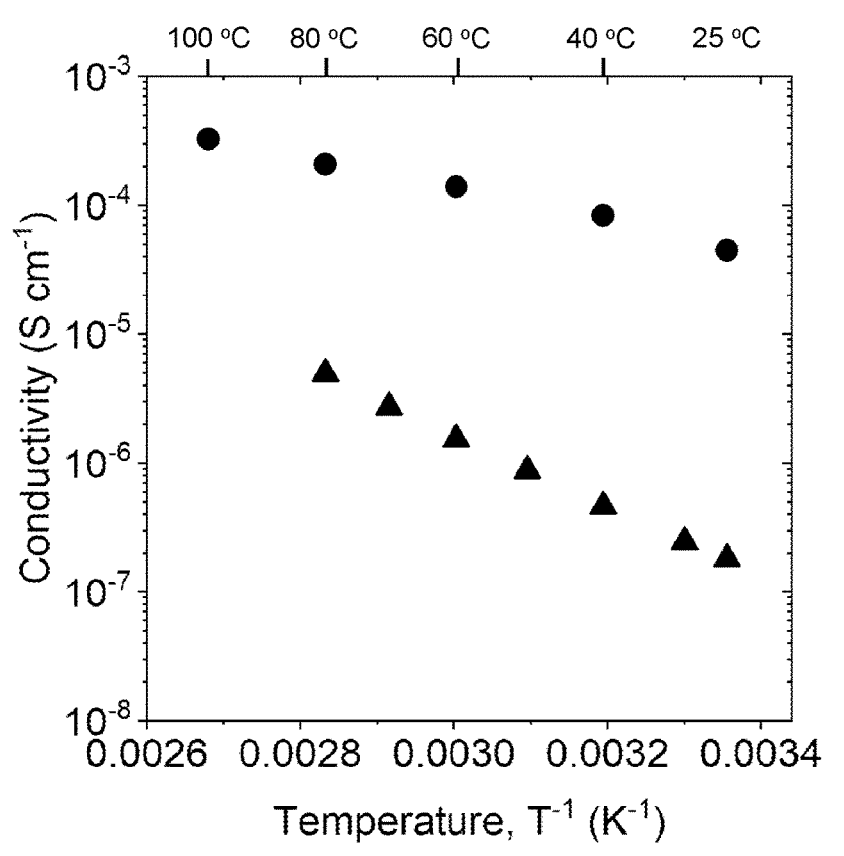
This work illustrates how defect-doping of FeOCl (which itself does not contain Li) can lead to dramatically increased
ion conductivity in combination with LiCl.
This work could have wide-ranging implications as it might point towards a wide unexplored product development parameter space
of defect-doped materials that do not contain Li that might substantially increase the Li-ion conductivity in combination with comparably low-cost Li-containing materials
(such as LiCl, this approach might allow for reduced lithium content in the solid electrolyte layer).
It would be very interesting to understand more about the underlying
Li-ion conduction mechanism. Do Li-ions exhibit increased mobility because defects in FeOCl
increase the mobility of chloride ions?
-
The premium version includes another two patent discussions, plus an Excel list with 50-100 commercially relevant recent patent families.
-
Get a quote to make better informed decisions.
-
Lithium-ion batteries – positive electrode
-
A carbonate precursor with the metal composition Ni0.27Co0.02Mn0.71 was heat-treated
(heating rate 5°C/min, keep for 10 h at 400°C, oxygen atmosphere), followed by mixing with Li2SO4 and Li2CO3,
molar ratio Li2SO4 / Li2CO3 = 0.067:1, ratio Li / (Ni+Co+Mn) = 1.40.
The resulting mixture was heat-treated (heating rate 5°C/min to 500°C, heating rate 1°C/min to 600°C, keep for 12 h at 600°C, dry air).
The resulting material was mixed with Li2CO3 to bring the ratio Li / (Ni+Co+Mn) to 1.47, followed by a heat treatment
(heating rate 1.5°C/min, keep for 10 h at 800°C, dry air). The resulting intermediate exhibits an Na content of 0.60 mass% (the origin of Na is unclear)
and an S content of 1.29 mass%. Subsequently, another heat treatment was carried out (700 °C, 10 h, dry air).
The resulting material was coated with an Al2O3 layer through ALD (atomic layer deposition, Al source: trimethylaluminium, 100-150°C,
Ar atmosphere, 12 cycles), resulting in a coating thickness of 1.7 nm and a BET specific surface area of 7.1 m2/g.
A 'capacity fade' value of 11.1% was determined (based on extrapolating the capacity difference between cycles 8 and 23), as compared to 38.8% for the
uncoated comparative material. The coated material exhibits an 8th cycle discharge capacity of 256.2 mAh/g.
This work illustrates a high-capacity, Mn-rich, Al2O3-coated positive electrode active material that presumably can be classified
as a Li-rich layered oxide (LRLO). It appears to be crucial that the manufacturing process involves employment of both Li2SO4 and Li2CO3 in the context
of multiple lithiation heat treatment steps.
It will be interesting to see if the cycling stability can be improved further in combination with liquid, semi-solid or solid electrolytes.
-
The premium version includes another two patent discussions, plus an Excel list with 50-100 commercially relevant recent patent families.
-
Get a quote to make better informed decisions.
-
Lithium-ion batteries – negative electrode (excluding Li metal electrodes)
-
Porous silicon fiber templates (PSFT) were prepared through a magnesiothermic reduction process
by heat-treating SiO2-containing fibers (made in turn
through sol-gel fiberization) in combination with magnesium metal and moderators (sodium chloride,
alumina beads, and/or tabular alumina), in an alumina crucible, metal crucible or rotary kiln (under argon).
The Si particle size was varied by varying reaction conditions (moderator composition, temperature, oven type), which affected
electrochemical performance as shown in the Figures below.
Pores in PSFT were coated with carbon through chemical vapor deposition (CVD, e.g. with acetylene gas),
resulting in 23.9-40.9 mass% carbon content.
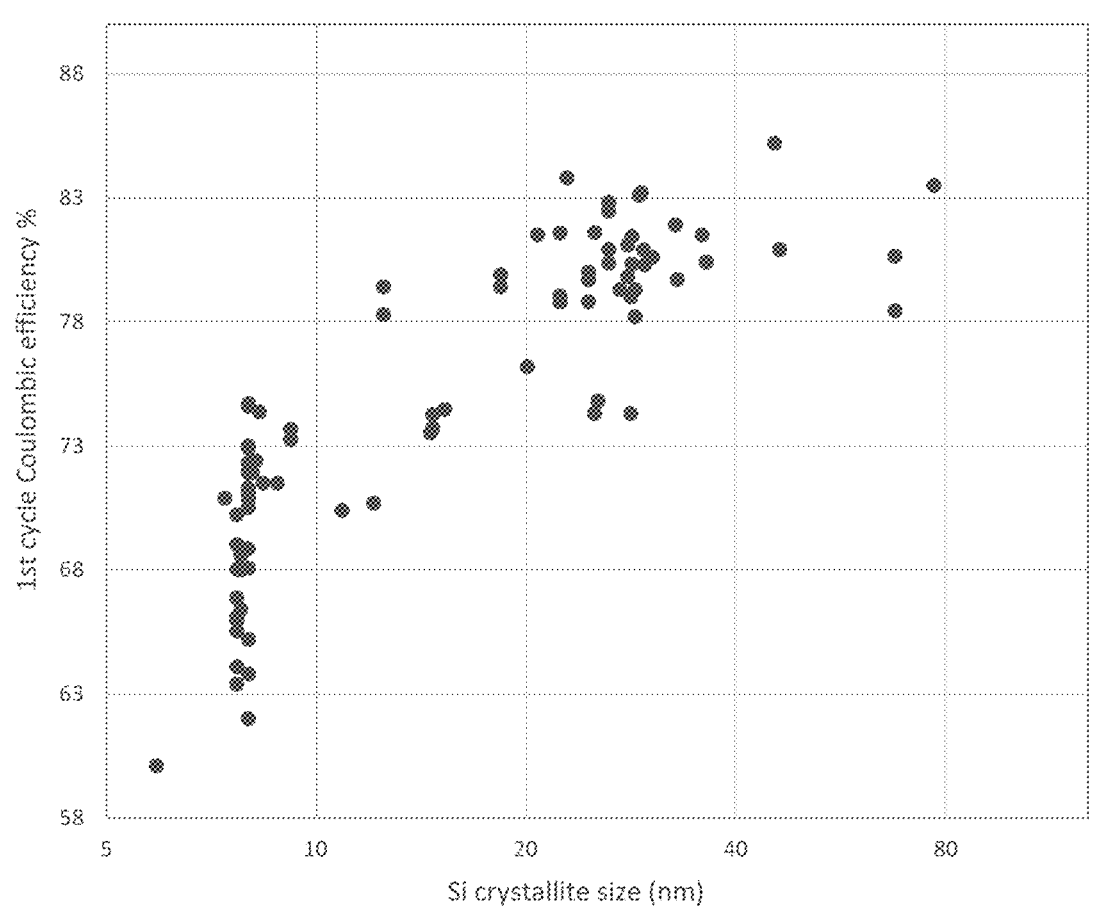
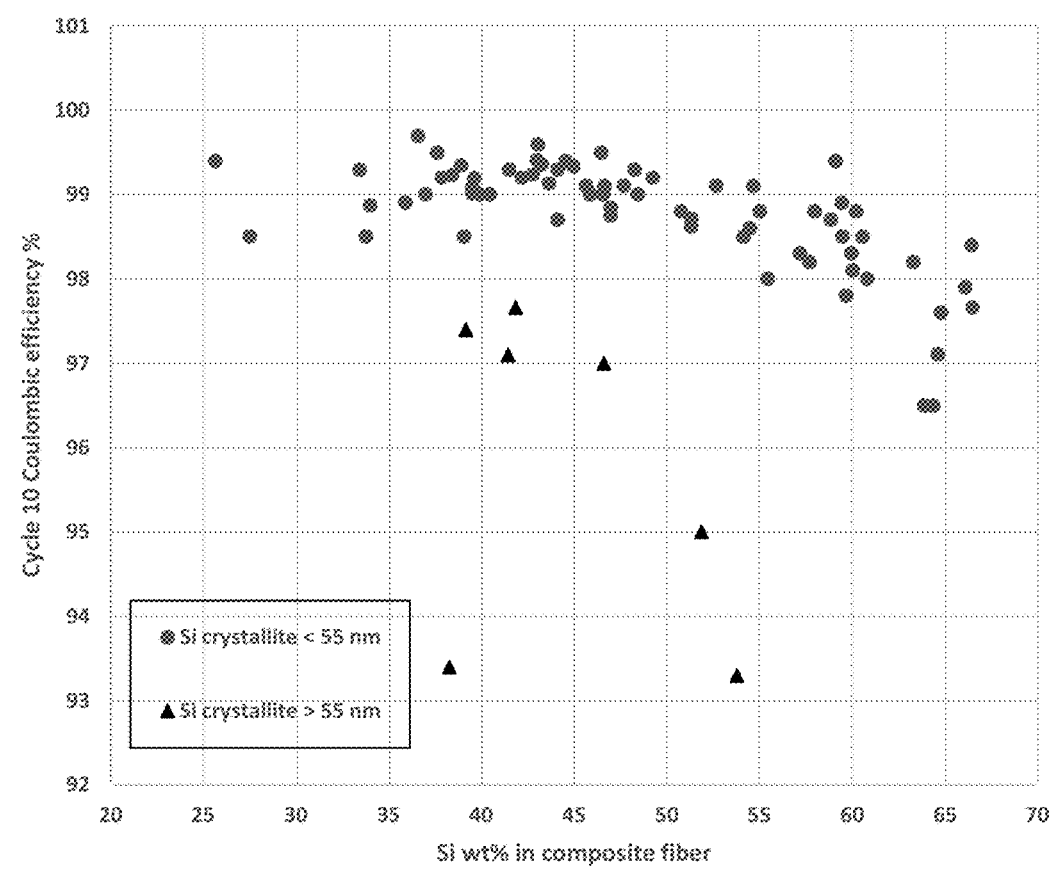
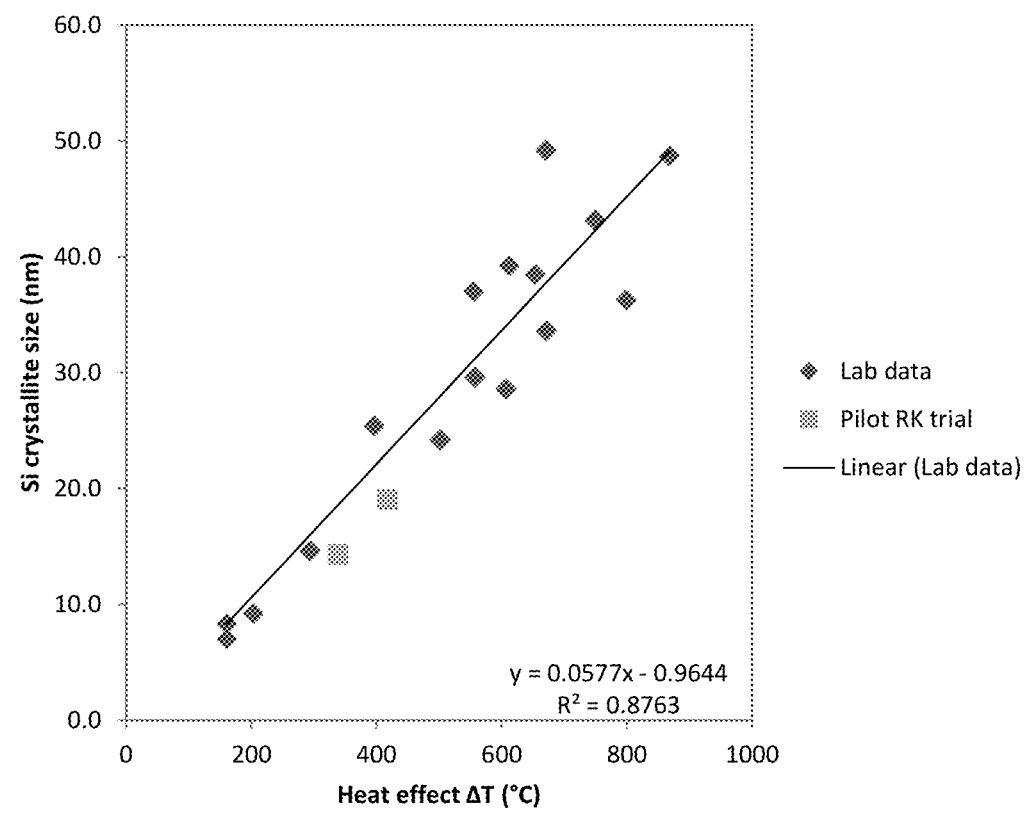
This work illustrates how Unifrax (renamed to Alkegen) has engaged in substantial process optimization efforts in the context of launching
its product (see press release).
It is nicely illustrated how the Si crystallite size can be controlled through process conditions, and that Si crystallites
ideally should not be too small and not too large (20-55 nm) in the context of the carbon-coated Si fiber architecture.
-
The premium version includes another two patent discussions, plus an Excel list with 50-100 commercially relevant recent patent families.
-
Get a quote to make better informed decisions.
-
Fuel cells (PEMFC / SOFC / PAFC / AEMFC) – electrochemically active materials
-
A mesoporous carbon support with a pore diameter of 4 nm (according to a BJH nitrogen adsorption / desorption measurement)
and an average particle size of 1.4 μm was mixed with dinitrodiammineplatinum nitric acid aqueous
solution, nickel nitrate hexahydrate, ruthenium nitrate and acetone (Pt:Ni:Ru molar ratio of 3:1:0.65).
This dispersion underwent a pressurization treatment at 40°C, reaching a supercritical state of 10 MPa for 60 min,
followed by depressurization to normal pressure.
The dispersion was vacuum-dried at 40°C for 2 h to eliminate acetone. Lastly, under a nitrogen atmosphere with 4 volume% hydrogen,
the dispersion was heated to 825°C for 3 h, reducing the metal precursors.
Platinum / nickel / ruthenium alloy particles with an average diameter of 3 nm formed within the carbon support pores,
yielding an electrode catalyst.
Catalyst coated membranes (CCM) prepared with the above catalyst in the cathode (anode catalyst: Pt on carbon black,
TEC10E50E, Tanaka Precious Metals) exhibit a mass-specific activity of 623 A/g-Pt at 0.85 V, as compared to
256 A/g-Pt at 0.85 V for Pt catalyst TEC10E50E (Tanaka Precious Metals).
This work illustrates how Pt / Ni / Ru particles with optimized particle size in mesoporous carbon support exhibit
favorable catalyst activity as compared to commercial Pt catalyst TEC10E50E (Tanaka Precious Metals).
-
The premium version includes another two patent discussions, plus an Excel list with 50-100 commercially relevant recent patent families.
-
Get a quote to make better informed decisions.
-
Triweekly patent lists for other categories (Excel files are included for premium users)
-
- Lithium metal containing batteries (excluding Li-S, Li-Air): XLSX
-
- Lithium-ion batteries – electrolytes – liquid: XLSX
-
- Lithium-ion batteries – separators: XLSX
-
- Lithium-sulfur batteries: XLSX
-
- Metal-air batteries: XLSX
-
- Na-ion batteries: XLSX
-
Prior patent updates
-
2023-09-19
|