-
Lithium-ion batteries – electrolytes – solid & semi-solid
-
Precursor powder Na3Zr2Si2PO12, Li2SO4 (4.5 eq., Li / Na molar ratio = 3)
and water were mixed in a hydrothermal
kettle (150°C, 16 h). After washing and drying (80°C), the resulting powder was mixed with additional Li2SO4
(4.5 eq.) and water (same mass as powder mixture), followed by ball-milling (zirconium balls, 3 h, 300 rpm).
The mixture was again mixed with water in a hydrothermal kettle (150°C, 16 h), yielding
Li3Zr2Si2PO12
powder that exhibits a 100% Na/Li ion exchange rate and an ion conductivity of 3.6 × 10-3 S/cm.
This work illustrates a procedure to exchange Na+ with Li+ in NASICON with 100% efficiency. Up-scaling at competitive
costs appears feasible and might be pursued by Ganfeng Lithium given its further patent filings related to
Li3Zr2Si2PO12.
The reported ion conductivity is consistent with an
academic article by Ning Zhao, Xiangxin Guo et al.,
who also report that
Li3Zr2Si2PO12
has been recognized as far back as the 1970ies as an attractive solid electrolyte candidate (studies by Goodenough et al.),
but that an efficient synthesis procedure has for a long time been elusive.
-
The premium version includes another two patent discussions, plus an Excel list with 50-100 commercially relevant recent patent families.
-
Lithium-ion batteries – positive electrode
-
An aqueous solution of NiSO4 · 6 H2O and MnSO4 · H2O (4 : 6 molar ratio) was mixed
with NaOH and NH4OH in a reactor (45°C, under nitrogen) to synthesize
Ni0.4Mn0.6(OH)2 (diameter: 3.5 μm). This precursor was coated by
mixing it with an aqueous solution of NiSO4 · 6 H2O (5 mol%), NaOH, and NH4OH, followed by drying (150°C, 14 h)
and a heat treatment
(550°C, 5 h, in air). This material was mixed with LiOH (Li / transition metals molar ratio = 1.22) and heat-treated
(900°C, 8 h, in oxygen) to obtain a gradient Ni-Mn layered oxide active material. Finally, the powder was mixed with B2O3 (1.5 mass%)
without further heat treatment.
In half-cells, this material exhibits a 0.1 C discharge capacity of 218 mAh/g and a first charge efficiency of 89.3%.
In full cells (graphite-based negative electrodes), the material exhibits a capacity retention of 86.7% after 500 cycles
(1 C charge / discharge).

This work illustrates well-balanced electrochemical characteristics in a Co-free layered, gradient Ni-Mn oxide material coated with B2O3.
-
The premium version includes another two patent discussions, plus an Excel list with 50-100 commercially relevant recent patent families.
-
Lithium-ion batteries – negative electrode (excluding Li metal electrodes)
-
Graphite particles (Dv50: ≈15 μm) were heated to 480°C in a vacuum stainless steel rotary furnace
(rotation speed: 8°/min, inclination: 15°). An electric field of 10 V was applied.
Monosilane and nitrogen (1 : 1 by volume) were continuously introduced (6 h).
The resulting Si-carbon composite material exhibits Si nanowires on the surface (diameter: 5 nm, length: 2.4 μm, no microscopic images are included
in the patent).
The material exhibits an Si content of 32.8%, a carbon content of 67.2%, and a BET specific surface area (SSA) of 210 m2/g.
In half-cells, the material exhibits a reversible capacity of
1,160 mAh/g, a first cycle efficiency of 91.8%, and a capacity retention after 100 cycles of 81% (0.1 C charge / discharge).
This work illustrates how Si nanowire formation on graphite from monosilane can be triggered in an electric field.
The first cycle efficiency is unexpectedly high in consideration of a BET SSA of 210 m2/g.
Such a high BET SSA might be acceptable in the context of cells with all-solid-state or semi-solid electrolytes,
while further work on substantially lowering the BET SSA might be necessary if use with liquid carbonate electrolyte
is targeted.
-
The premium version includes another two patent discussions, plus an Excel list with 50-100 commercially relevant recent patent families.
-
Fuel cells (PEMFC / SOFC / PAFC / AEMFC) – electrochemically active materials
-
BaSc0.8Mo0.2O3–δ (δ: 0-0.5) was synthesized by mixing BaCO3, Sc2O3
and MoO3,
followed by a heat treatment (900°C, 12 h). The resulting material was exposed to repeated dry and wet (ethanol) grinding (30-60 min), molding into pellets (150 MPa uniaxial
pressure) and sintering (1,500°C, 12 h).
As shown in the Figure below, this material exhibits a favorable proton conductivity, which makes the material
attractive for use in SOFC (solid-oxide fuel cells).
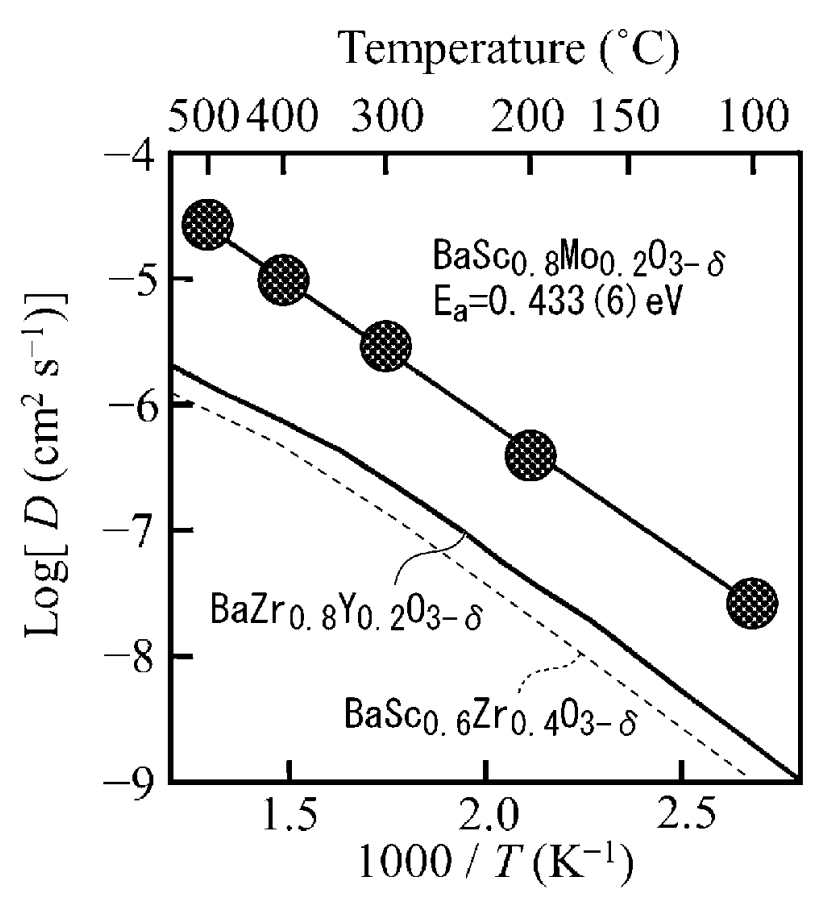
This work illustrates favorable proton conductivity in a Ba- & Sc-containing material. A range of other materials were studied base
well that are based on elements with varying scarcity.
-
The premium version includes another two patent discussions, plus an Excel list with 50-100 commercially relevant recent patent families.
-
Triweekly patent lists for other categories (Excel files are included for premium users)
-
- Lithium metal containing batteries (excluding Li-S, Li-Air): XLSX
-
- Lithium-ion batteries – electrolytes – liquid: XLSX
-
- Lithium-ion batteries – separators: XLSX
-
- Lithium-sulfur batteries: XLSX
-
- Metal-air batteries: XLSX
-
- Na-ion batteries: XLSX
-
Prior patent updates
-
2024-03-26
-
2024-03-05
-
2024-02-13
-
2024-01-23
-
2024-01-02
|