-
Lithium-ion batteries – electrolytes – solid & semi-solid
-
A device as shown in the Figure is claimed that consists of:
-
An LCO (LiCoO2) positive electrode active material network formed by depositing a binder-containing 'green sheet', followed by sintering
(binder volatilization / formation of pores around LCO network).
-
Application of a coating on the LCO network, such as an LiNbO3, Li2ZrO3, Li2SiO3
or Al2O3 coating, obtained through a wet coating method or ALD (atomic layer deposition).
-
Filling of the positive electrode pores with a combination of an ion-conductive polymer (such as poly[oxymethylene-oligo(oxyethylene)]),
a lithium salt (such as LiTFSI, lithium bis(-trifluoromethanesulfonyl)imide), and an ion-conducting oxide (or sulfide) with higher ion-conductivity
than the polymer / salt mixture (at least one order of magnitude higher).
-
LATP solid electrolyte layer (presumably obtained through binder-containing slurry deposition, with a thickness
of ≤10 μm).
-
Interlayer, such as LiPON, deposited on LATP layer through ALD (about 10 nm thickness, protects LATP from being in contact with lithium metal).
-
Lithium metal negative electrode.
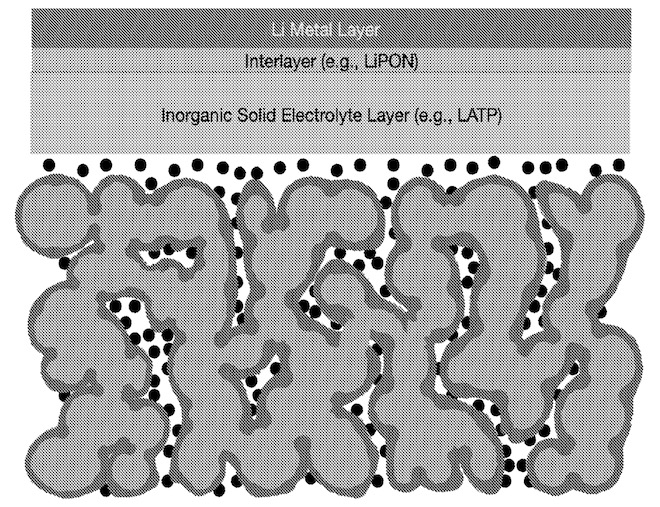
Although limited experimental details and no electrochemical results are included in this patent application, this work illustrates
important and underexplored concepts, including the attractive possibility of forming a continuous positive electrode active material
network (presumably with favorable internal ion and electrical conductivity) along with a tailored electrode pore size distribution to allow for
favorable mechanical characteristics (compensation of volume change) and ion diffusion.
Coating of LATP with LiPON combines the advantages of LATP (well-rounded characteristics including raw material abundance, except
for instability in contact with lithium metal) with LiPON (favorable interface characteristics and stability in contact with lithium metal,
but limited ion conductivity).
Presumably, cells will first be targeted towards high value niches that require high safety, high energy and high power.
-
The premium version includes another two patent discussions, plus an Excel list with 50-100 commercially relevant recent patent families.
-
Lithium-ion batteries – positive electrode
-
NMC811 was produced by blending Ni / Mn / Co metal powders and Li2CO3 (1 mol% excess).
The mix was ground, placed in an alumina crucible, and heated in a tube furnace (600°C for 3 h, then 920°C for 20 h, then 870°C for 5 h,
10°C/min heating rate, oxygen atmosphere).
A SEM image (see Figure) reveals a single-crystal morphology with smooth surfaces. The material exhibits 1.29% Ni / Li cation mixing.
Charge-discharge tests exhibit a reversible capacity of 191.1 mAh/g, 1st cycle losses of 13.4% and 93.6% capacity retention after 25 cycles (see Figure).
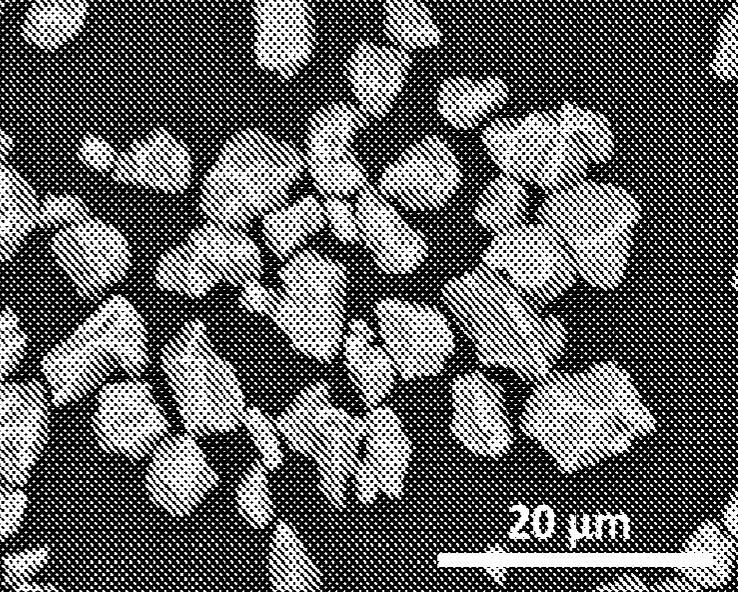
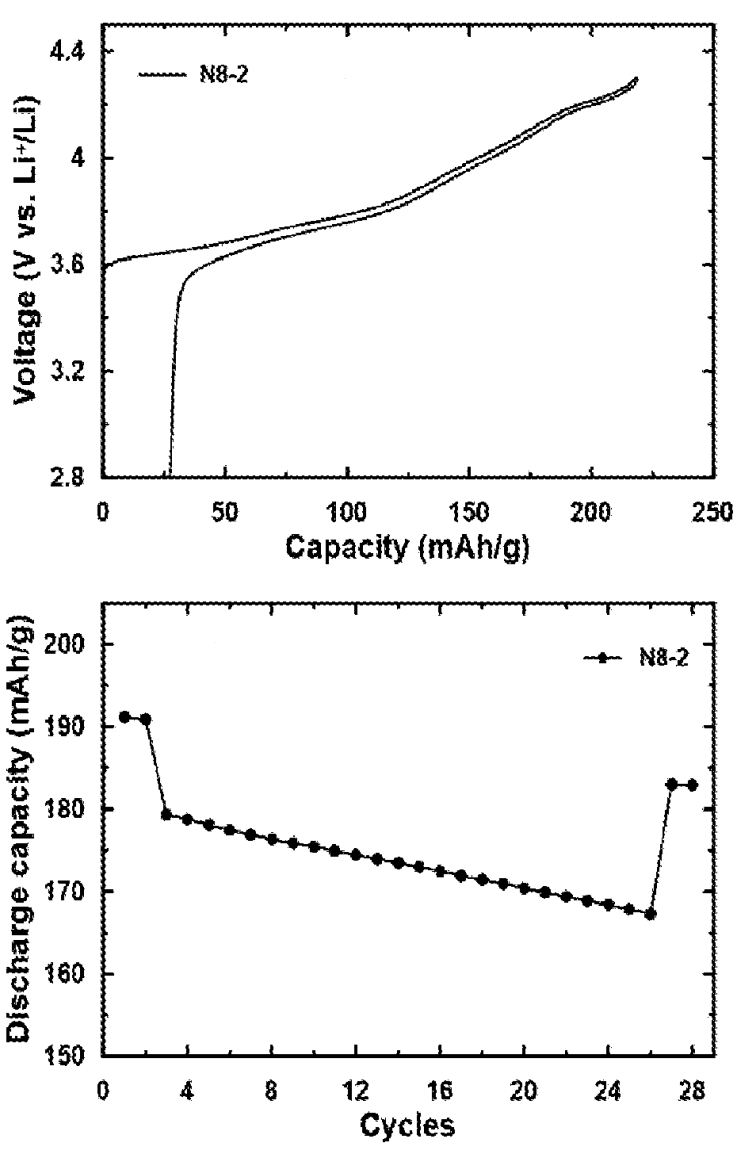
This work suggests that single-crystal NMC811 powder fabrication from metal precursors is possible with a straightforward,
dry procedure as long as the multi-step heat-treatment steps are carefully optimized.
It will be interesting to see if further improvements to reversible capacity, 1st cycle losses and cycling stability are feasible,
e.g. by employing doping and coating approaches.
-
The premium version includes another two patent discussions, plus an Excel list with 50-100 commercially relevant recent patent families.
-
Lithium-ion batteries – negative electrode (excluding Li metal electrodes)
-
Single-walled carbon nanotubes (SWCNT, modified with carboxyl functional groups), ammonium bicarbonate,
and phenolic resin were mixed (2:5:93 by mass)
and stirred (60°C, 1 h), followed by pre-curing (80°C, 10 h), followed by airflow milling to
achieve a Dv50 particle size of 7 μm. This powder was solidified (160°C, 15 h, nitrogen atmosphere), followed by
carbonization (1,100°C, 4 h, nitrogen atmosphere), resulting in a SWCNT / porous carbon composite matrix.
Si was deposited into pores of this matrix (CVD, chemical vapor deposition, monosilane / hydrogen, 500°C, 10 h, 10 nm Si domain size), followed
by coating with carbon (CVD, ethene / nitrogen, 800°C, 0.5 h).
The resulting Si-carbon composite material exhibits a BET specific surface area of 4 m2/g, and a cycling stability of 96.1%
(after 300 cycles at 45°C, 1 C charge / discharge, negative electrodes based on Si-carbon composite / graphite / styrene-butadiene rubber /
polyacrylic acid / Na-carboxymethyl cellulose / Super P carbon black / carbon nanotubes = 10 : 85 : 2 : 1 : 1 : 0.7 : 0.3 by mass, NMC811-based positive electrodes),
as compared to 88.3% for a comparative cell based on a Si-carbon composite material prepared without carbon nanotubes.
This work illustrates how the incorporation of carboxylate-functionalized SWCNT into the carbon matrix of Si-carbon composite materials results in substantially
improved cycling stability, which is attributed by the inventors to an improved electrical conductivity and mechanical stability of the carbon matrix.
-
The premium version includes another two patent discussions, plus an Excel list with 50-100 commercially relevant recent patent families.
-
Fuel cells (PEMFC / SOFC / PAFC / AEMFC) – electrochemically active materials
-
A catalyst layer was prepared by coating a slurry based of TEC10E50E (platinum on Ketjen Black, 46.8 mass% platinum)
and 20 mass% Nafion (solvents: ethanol / water) on a PTFE transfer sheet.
The coated PTFE sheet was placed on a hot plate at 100°C to quickly evaporate the solvent.
Membrane-electrode assemblies (MEA) were produced with catalyst sheets dried quickly at 100°C and dried at 25°C.
8.03 W/mg-Pt was
measured for the MEA with the catalyst layer dried at 100°C (Pt loading: 0.144 mg-Pt/cm2) as compared to 5.98 W/mg-Pt
for the MEA with the catalyst
layer dried at 25°C (Pt loading: 0.16 mg-Pt/cm2).
This work illustrates how the velocity with which catalyst layers used in PEMFC devices are dried can substantially influence
power performance.
-
The premium version includes another two patent discussions, plus an Excel list with 50-100 commercially relevant recent patent families.
-
Triweekly patent lists for other categories (Excel files are included for premium users)
-
- Lithium metal containing batteries (excluding Li-S, Li-Air): XLSX
-
- Lithium-ion batteries – electrolytes – liquid: XLSX
-
- Lithium-ion batteries – separators: XLSX
-
- Lithium-sulfur batteries: XLSX
-
- Metal-air batteries: XLSX
-
- Na-ion batteries: XLSX
-
Prior patent updates
-
2024-03-05
-
2024-02-13
-
2024-01-23
-
2024-01-02
-
2023-12-12
|