-
Lithium-ion batteries – electrolytes – solid & semi-solid
-
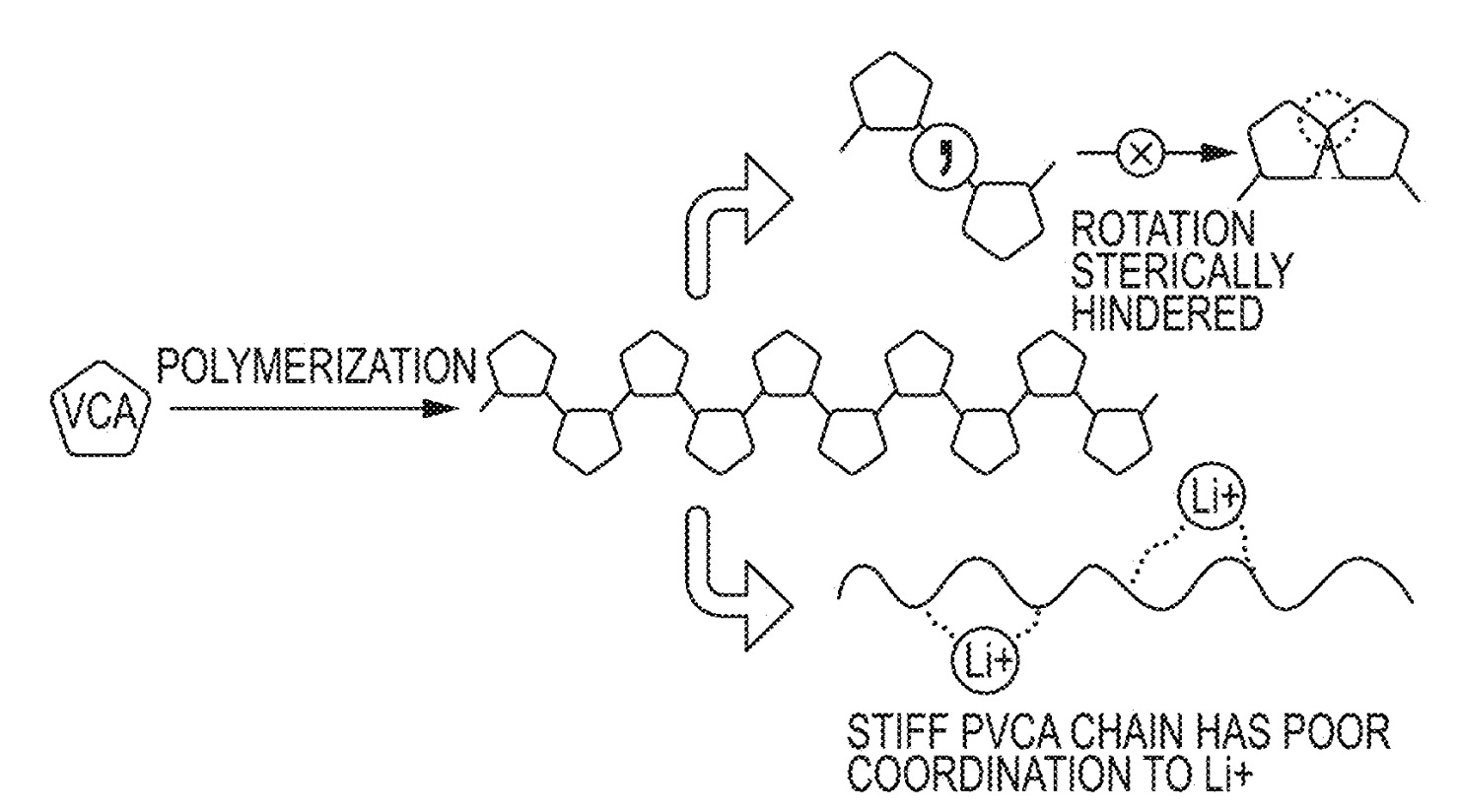
In this patent application, the hypothesis is made that polyvinylene carbonate (PVCA) polymers with too limited flexibility
due to steric hindrance exhibit limited ionic conductivity (top Figure), while PVCA polymers that exhibit a higher conformational
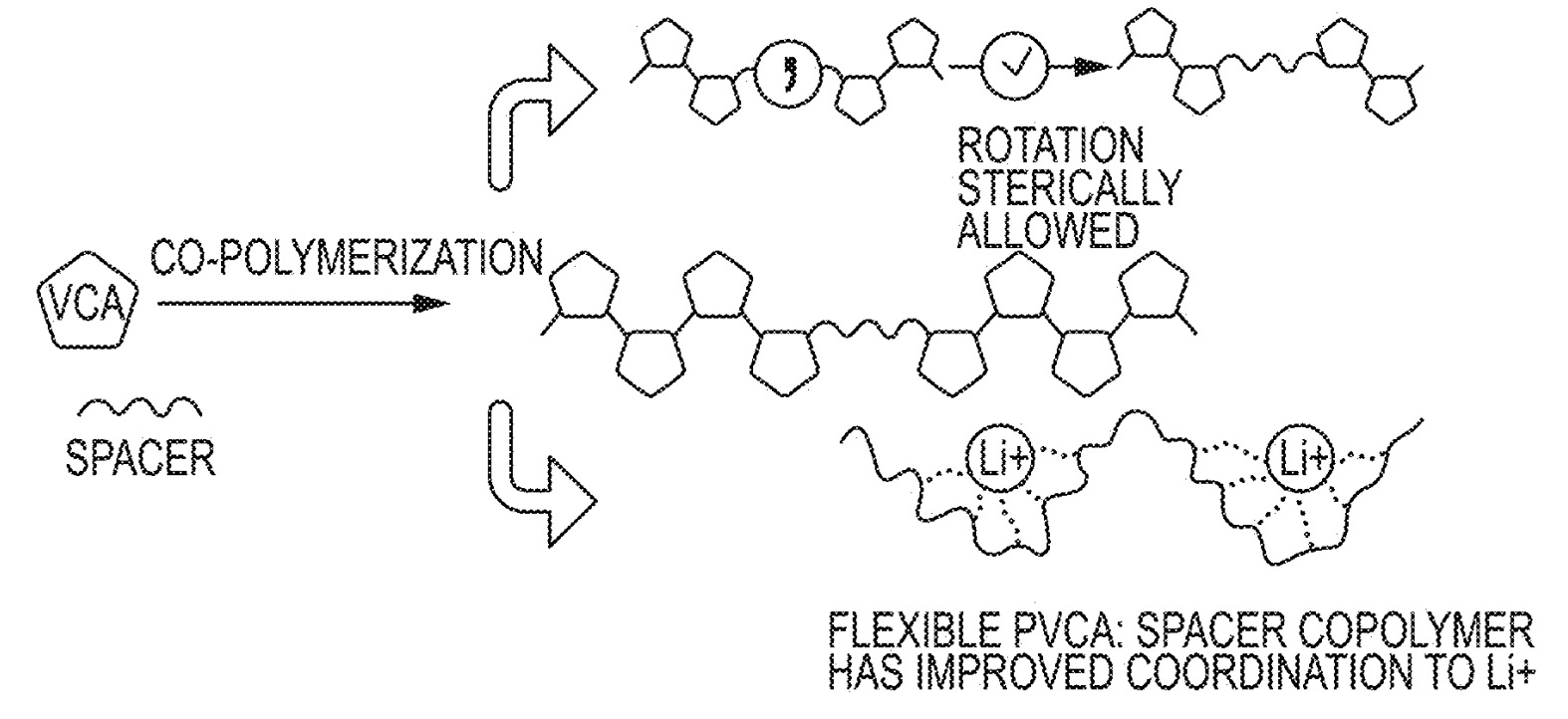
flexibility can undergo supramolecular chelation interactions with Li-ions that result in favorable ionic conductivity (middle Figure).
This hypothesis was validated through the synthesis of various co-polymers based on vinylene carbonate (VC),
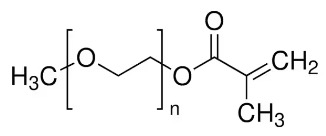
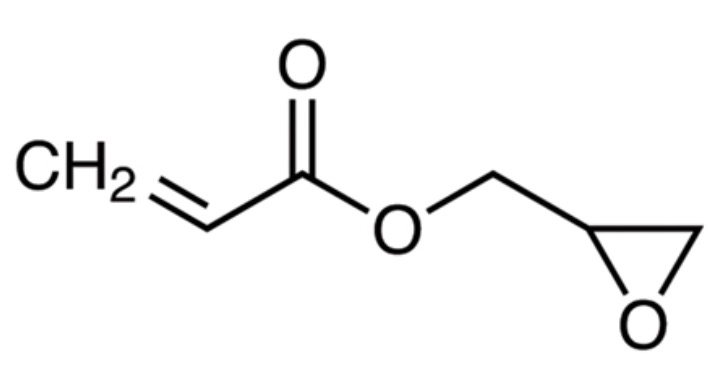
poly(ethylene glycol) methyl ether methacrylate (MW: 360, PEGMA360, bottom Figure), glycidal acrylate (GLA, bottom Figure)
and dimethyl vinylphosphonate (DMVP, bottom Figure).
The best ionic conductivity for a mixture that allows for blade-casting of of a free-standing film is based on
a polymer / LiTFSI (lithium bis(trifluoromethane)sulfonimide) mixture (1 : 1.165 by mass) and a VC / DMVP monomer ratio of
18 : 1, 2.05 × 10-4 S/cm.
This film exhibits an oxidative stability of 5.5 V vs. Li+/Li.
An ionic conductivity of 6.8 × 10-4 S/cm was measured for a polymer / LiTFSI mixture (1 : 2.246 by mass)
in which the polymer was prepared based on a VC / PEGMA360 / glycidal acrylate molar ratio of 5 : 1.67 : 1. However,
due to the waxy characteristics of this mixture, no free-standing film was obtained.
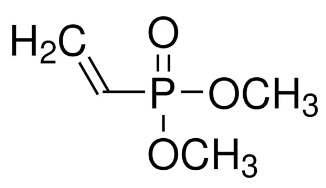
This work illustrates how the incorporation of phosphonate groups into polycarbonate polymers increases ionic conductivity.
The hypothesis described in this work potentially reveals how the achievement of a favorable ionic conductivity and of
mechanical properties that enable the formation of a free-standing film conflict with each other.
For this reason, it
is important to evaluate approaches with which waxy or brittle electrolyte mixtures with favorable ionic conductivity can be mechanically stabilized,
such as through incorporation into a porous framework.
-
The premium version includes another two patent discussions, plus an Excel list with 50-100 commercially relevant recent patent families.
-
Lithium-ion batteries – positive electrode
-
Li2CO3, NiCO3 ∙ 2 Ni(OH)2 ∙ 4 H2O
and MnCO3 were mixed at a molar ratio of 1.16 : 0.24 : 0.6, followed by a heat treatment
(1,050°C, 12 h, under air). Single-crystal Li1.16Ni0.24Mn0.6O2 positive electrode active material was obtained
(see Figure).
In half-cells at 45°C, this material exhibits a discharge capacity of 277.9 mAh/g and a first cycle efficiency of 87.0%
(voltage window: 2-4.65 V vs. Li+/Li).

This work illustrates a promising discharge capacity and first cycle efficiency for an overlithiated Mn-Ni single-crystal positive electrode material with
an unusually high Mn content.
It appears that the selection of Li and Mn carbonate precursors in combination with mixed carbonate / hydroxide NiCO3 ∙ 2 Ni(OH)2 ∙ 4 H2O
plays a role to obtain
single-crystalline as opposed to polycrystalline materials.
Because of the raw material composition and the dry manufacturing process, this product could exhibit favorable manufacturing costs.
-
The premium version includes another two patent discussions, plus an Excel list with 50-100 commercially relevant recent patent families.
-
Lithium-ion batteries – negative electrode (excluding Li metal electrodes)
-
Artificial graphite, (40 mass% particles with D50 = 5 μm, 60 mass% particles with D50 = 25 μm)
was mixed in water with dodecylamine (mass ratio 100 : 20).
After standing for 2 h, solid-liquid separation was performed to retain the liquid phase.
This dispersion was centrifuged at 10,000 rpm, and the solid phase was retained,
yielding a positively charged carbon-based material with amino / ammonium groups on the surface.
Silicon (D50 = 5 μm) and sodium cholate were mixed in water (mass ratio 100 : 20).
After standing for 2 hours, solid-liquid separation was performed to retain the liquid phase.
This dispersion was centrifuged at 10,000 rpm, and the solid phase was retained,
yielding a negatively charged silicon-based material with carboxyl / carboxylate groups on the surface.
Both materials were mixed to obtain a negative electrode active material (mass ratio of 50:50, no mixing details identified).
Negative electrode formulation: negative electrode active material / acetylene black / sodium carboxymethyl cellulose (CMC) /
styrene butadiene rubber (SBR) = 96 : 2 : 1 : 1 by mass.
In full cells (NMC811-based positive electrodes), a 1 C / 0.33 C discharge capacity retention of 91.0% was observed as compared to 86.9%
for comparative cells with negative electrodes obtained through simple Si / graphite mixing (without dodecylamine, sodium cholate).
This work illustrates how the fast discharge performance of graphite / Si mixtures can be improved upon coating each
of these materials with positively and negatively charged surfactants, respectively.
This approach could also be helpful with graphite / Si-carbon composite mixtures.
-
The premium version includes another two patent discussions, plus an Excel list with 50-100 commercially relevant recent patent families.
-
Fuel cells (PEMFC / SOFC / PAFC / AEMFC) – electrochemically active materials
-
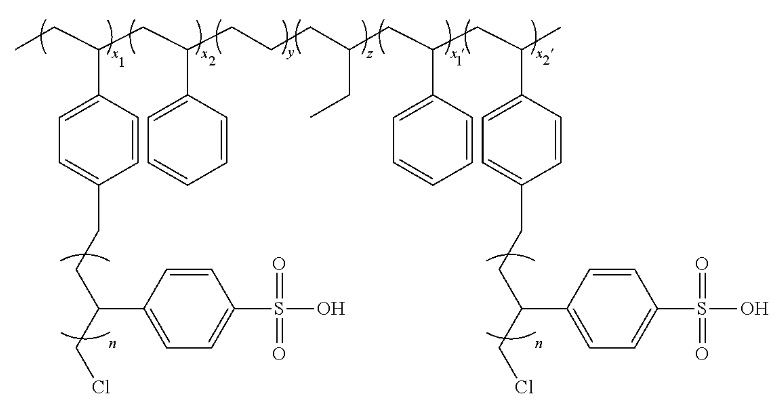
A copolymer with hydrophilic and hydrophobic moieties was prepared (see Figure, n: 12, x1, x1', x2, x2': ≈50, y, z: ≈100) that forms a star-like supramolecular structure.
A corresponding PEMFC membrane exhibits a proton conductivity of 3.17 × 10-1 S/cm at 90% relative humidity, and of
2.0 × 10-2 S/cm at 50% relative humidity.
A: hydrophilic moiety
B: hydrophobic moiety
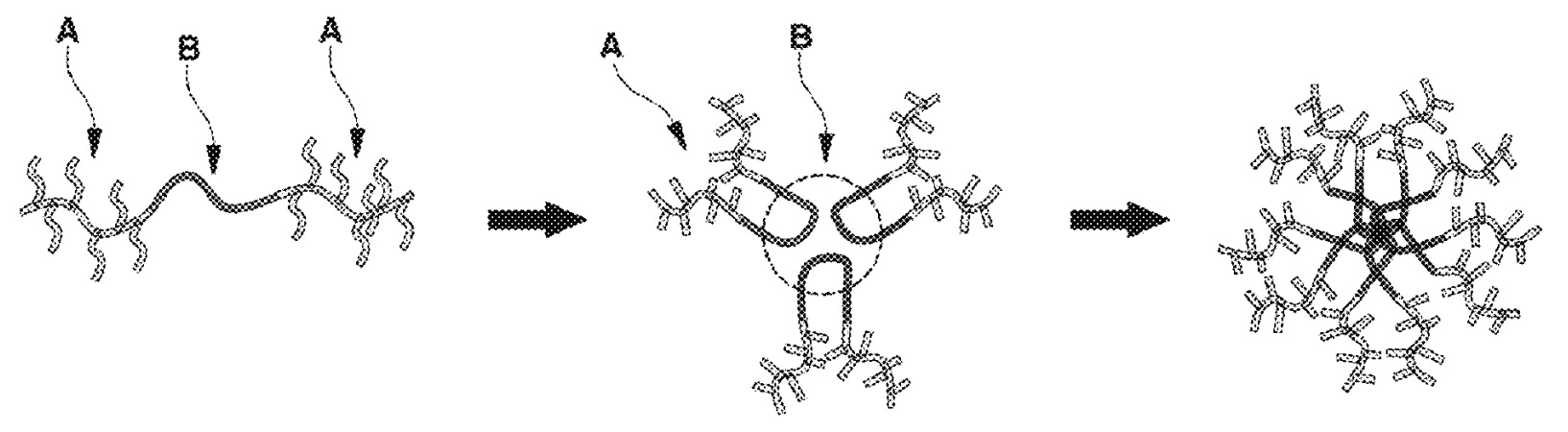
This work illustrates favorable ion conductivity with a fluoride-free supramolecular ionomer.
-
The premium version includes another two patent discussions, plus an Excel list with 50-100 commercially relevant recent patent families.
-
Triweekly patent lists for other categories (Excel files are included for premium users)
-
- Lithium metal containing batteries (excluding Li-S, Li-Air): XLSX
-
- Lithium-ion batteries – electrolytes – liquid: XLSX
-
- Lithium-ion batteries – separators: XLSX
-
- Lithium-sulfur batteries: XLSX
-
- Metal-air batteries: XLSX
-
- Na-ion batteries: XLSX
-
Prior patent updates
-
2024-05-28
-
2024-05-07
-
2024-04-16
-
2024-03-26
-
2024-03-05
|